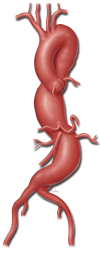
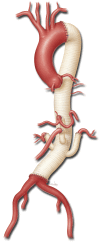
Thoracoabdominal aortic aneurysm repair with a branched graft
- PMID: 23977524
- PMCID: PMC3741776
- DOI: 10.3978/j.issn.2225-319X.2012.08.05
Thoracoabdominal aortic aneurysm repair with a branched graft
Figures


References
-
- De Rango P, Estrera AL, Miller C, 3rd, et al. Operative outcomes using a side-branched thoracoabdominal aortic graft (STAG) for thoraco-abdominal aortic repair. Eur J Vasc Endovasc Surg 2011;41:41-7 - PubMed
-
- Kouchoukos NT, Masetti P, Castner CF. Use of presewn multiple branched graft in thoracoabdominal aortic aneurysm repair. J Am Coll Surg 2005;201:646-9 - PubMed
-
- Kulik A, Castner CF, Kouchoukos NT. Patency and durability of presewn multiple branched graft for thoracoabdominal aortic aneurysm repair. J Vasc Surg 2010;51:1367-72 - PubMed
-
- Dardik A, Perler BA, Roseborough GS, et al. Aneurysmal expansion of the visceral patch after thoracoabdominal aortic replacement: an argument for limiting patch size? J Vasc Surg 2001;34:405-9; discussion 410 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
