Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia
- PMID: 23977987
- PMCID: PMC4133907
- DOI: 10.5483/bmbrep.2013.46.8.237
Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia
Abstract
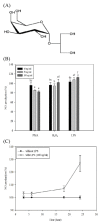
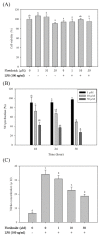
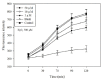
Inflammatory conditions mediated by activated microglia lead to chronic neuro-degenerative diseases such as Alzheimer's, Parkinson's, and Huntington's diseases. This study was conducted to determine the effect of floridoside isolated from marine red algae Laurencia undulata on LPS (100 ng/ml) activated inflammatory responses in BV-2 microglia cells. The results show that floridoside has the ability to suppress pro-inflammatory responses in microglia by markedly inhibiting the production of nitric oxide (NO) and reactive oxygen species (ROS). Moreover, floridoside down-regulated the protein and gene expression levels of iNOS and COX-2 by significantly blocking the phosphorylation of p38 and ERK in BV-2 cells. Collectively, these results indicate that floridoside has the potential to be developed as an active agent for the treatment of neuro-inflammation.
Figures

References
-
- Lehnardt S., Massillon L., Follett P., Jensen F. E., Ratan R., Rosenberg P. A., Volpe J. J., Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. U. S. A. (2003);100:8514–8519. doi: 10.1073/pnas.1432609100. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

