Influenza virus hemagglutinin stalk-based antibodies and vaccines
- PMID: 23978327
- PMCID: PMC3804342
- DOI: 10.1016/j.coviro.2013.07.007
Influenza virus hemagglutinin stalk-based antibodies and vaccines
Abstract
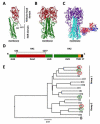
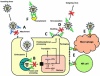
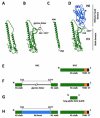
Antibodies against the conserved stalk domain of the hemagglutinin are currently being discussed as promising therapeutic tools against influenza virus infections. Because of the conservation of the stalk domain these antibodies are able to broadly neutralize a wide spectrum of influenza virus strains and subtypes. Broadly protective vaccine candidates based on the epitopes of these antibodies, for example, chimeric and headless hemagglutinin structures, are currently under development and show promising results in animals models. These candidates could be developed into universal influenza virus vaccines that protect from infection with drifted seasonal as well as novel pandemic influenza virus strains therefore obviating the need for annual vaccination, and enhancing our pandemic preparedness.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures


References
-
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, et al. Human Infection with a Novel Avian-Origin Influenza A (H7N9) Virus. N Engl J Med. 2013 - PubMed
-
- Palese P, Shaw ML. Fields’ virology. Lippincott Williams & Wilkins; Philadelphia: 2007.
-
- Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis. 2011;204:1879–1885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

