Gremlin-1 associates with fibrillin microfibrils in vivo and regulates mesothelioma cell survival through transcription factor slug
- PMID: 23978876
- PMCID: PMC3759128
- DOI: 10.1038/oncsis.2013.29
Gremlin-1 associates with fibrillin microfibrils in vivo and regulates mesothelioma cell survival through transcription factor slug
Abstract
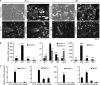
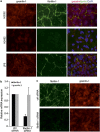
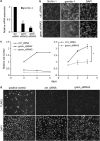
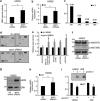
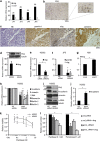
Malignant mesothelioma is a form of cancer that is highly resistant to conventional cancer therapy for which no major therapeutic advances have been introduced. Here, we identify gremlin-1, a known bone morphogenetic protein inhibitor crucial for embryonic development, as a potential therapeutic target for mesothelioma. We found high expression levels of gremlin-1 in the mesothelioma tumor tissue, as well as in primary mesothelioma cells cultured from pleural effusion samples. Downregulation of gremlin-1 expression by siRNA-mediated silencing in a mesothelioma cell line inhibited cell proliferation. This was associated with downregulation of the transcription factor slug as well as mesenchymal proteins linked to cancer epithelial-to-mesenchymal transition. Further, resistance to paclitaxel-induced cell death was associated with high gremlin-1 and slug expression. Treatment of gremlin-1-silenced mesothelioma cells with paclitaxel or pemetrexed resulted in efficient loss of cell survival. Finally, our data suggest that concomitant upregulation of fibrillin-2 in mesothelioma provides a mechanism for extracellular localization of gremlin-1 to the tumor microenvironment. This was supported by the demonstration of interactions between gremlin-1, and fibrillin-1 and -2 peptides as well as by colocalization of gremlin-1 to fibrillin microfibrils in cells and tumor tissue samples. Our data suggest that gremlin-1 is also a potential target for overcoming drug resistance in mesothelioma.
Figures



References
-
- Mossman BT, Bignon J, Corn M, Seaton A, Gee JB. Asbestos: scientific developments and implications for public policy. Science. 1990;247:294–301. - PubMed
-
- Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992;34:718–721. - PubMed
-
- Huuskonen MS, Rantanen J. Finnish Institute of Occupational Health (FIOH): prevention and detection of asbestos-related diseases, 1987-2005. Am J Ind Med. 2006;49:215–220. - PubMed
-
- Vorobiof DA, Mafafo K. Malignant pleural mesothelioma: medical treatment update. Clin Lung Cancer. 2009;10:112–117. - PubMed
-
- Dormoy V, Jacqmin D, Lang H, Massfelder T. From development to cancer: lessons from the kidney to uncover new therapeutic targets. Anticancer Res. 2012;32:3609–3617. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

