Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases
- PMID: 23979137
- PMCID: PMC3789967
- DOI: 10.1074/jbc.M113.488213
Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases
Abstract
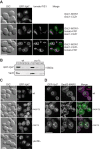
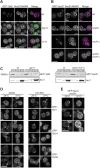
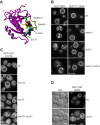
Membrane fusion at eukaryotic organelles is initiated by Rab GTPases and tethering factors. Rabs in their GDP-bound form are kept soluble in the cytoplasm by the GDP dissociation inhibitor (GDI) chaperone. Guanine nucleotide exchange factors (GEFs) are found at organelles and are critical for Rab function. Here, we surveyed the overall role of GEFs in Rab localization. We show that GEFs, but none of the proposed GDI displacement factors, are essential for the correct membrane localization of yeast Rabs. In the absence of the GEF, Rabs lost their primary localization to the target organelle. Several Rabs, such as vacuolar Ypt7, were found at the endoplasmic reticulum and thus were still membrane-bound. Surprisingly, a Ypt7 mutant that undergoes facilitated nucleotide exchange localized to vacuoles independently of its GEF Mon1-Ccz1 and rescued vacuole morphology. In contrast, wild-type Ypt7 required its GEF for localization and to counteract the extraction by GDI. Our data agree with the emerging model that GEFs are critical for Rab localization but raise the possibility that additional factors can contribute to this process.
Keywords: GAP; GDF; Guanine Nucleotide Exchange Factor (GEF); Membrane Fusion; Rab; Rab Proteins; Subcellular Organelles.
Figures


References
-
- Yu I.-M., Hughson F. M. (2010) Tethering factors as organizers of intracellular vesicular traffic. Annu. Rev. Cell Dev. Biol. 26, 137–156 - PubMed
-
- Bröcker C., Engelbrecht-Vandré S., Ungermann C. (2010) Multisubunit tethering complexes and their role in membrane fusion. Curr. Biol. 20, R943–R952 - PubMed
-
- Itzen A., Goody R. S. (2011) GTPases involved in vesicular trafficking: structures and mechanisms. Semin. Cell Dev. Biol. 22, 48–56 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

