Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis
- PMID: 23979159
- PMCID: PMC3755983
- DOI: 10.1172/JCI45361
Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis
Abstract
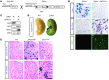
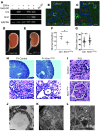
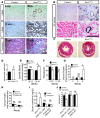
Acute kidney injury predisposes patients to the development of both chronic kidney disease and end-stage renal failure, but the molecular details underlying this important clinical association remain obscure. We report that kidney injury molecule-1 (KIM-1), an epithelial phosphatidylserine receptor expressed transiently after acute injury and chronically in fibrotic renal disease, promotes kidney fibrosis. Conditional expression of KIM-1 in renal epithelial cells (Kim1(RECtg)) in the absence of an injury stimulus resulted in focal epithelial vacuolization at birth, but otherwise normal tubule histology and kidney function. By 4 weeks of age, Kim1(RECtg) mice developed spontaneous and progressive interstitial kidney inflammation with fibrosis, leading to renal failure with anemia, proteinuria, hyperphosphatemia, hypertension, cardiac hypertrophy, and death, analogous to progressive kidney disease in humans. Kim1(RECtg) kidneys had elevated expression of proinflammatory monocyte chemotactic protein-1 (MCP-1) at early time points. Heterologous expression of KIM-1 in an immortalized proximal tubule cell line triggered MCP-1 secretion and increased MCP-1-dependent macrophage chemotaxis. In mice expressing a mutant, truncated KIM-1 polypeptide, experimental kidney fibrosis was ameliorated with reduced levels of MCP-1, consistent with a profibrotic role for native KIM-1. Thus, sustained KIM-1 expression promotes kidney fibrosis and provides a link between acute and recurrent injury with progressive chronic kidney disease.
Figures





Comment in
-
Renal fibrosis: KIM-1 expression links kidney injury with CKD in mice.Nat Rev Nephrol. 2013 Nov;9(11):627. doi: 10.1038/nrneph.2013.194. Epub 2013 Sep 17. Nat Rev Nephrol. 2013. PMID: 24042465 No abstract available.
-
Biomarkers: more than just markers!Nephrol Dial Transplant. 2015 Jan;30(1):33-8. doi: 10.1093/ndt/gfu085. Epub 2014 Apr 21. Nephrol Dial Transplant. 2015. PMID: 24755324
References
-
- Clarkson MR, Friedewald JJ, Eustace H, Rabb H. Brenner and Rector’s The Kidney. 2008. Acute kidney injury. In: Brenner BM, ed. pp. 943–986. 8th ed. Philadelphia, Pennsylvania, USA: Saunders Elsevier;
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK088923/DK/NIDDK NIH HHS/United States
- R37 DK039773/DK/NIDDK NIH HHS/United States
- R03 DK084316/DK/NIDDK NIH HHS/United States
- DK088923/DK/NIDDK NIH HHS/United States
- R37 DK054364/DK/NIDDK NIH HHS/United States
- K08 DK073628/DK/NIDDK NIH HHS/United States
- R01 DK054364/DK/NIDDK NIH HHS/United States
- DK84316/DK/NIDDK NIH HHS/United States
- DK72381/DK/NIDDK NIH HHS/United States
- K01 DK090105/DK/NIDDK NIH HHS/United States
- R01 DK072381/DK/NIDDK NIH HHS/United States
- DK39773/DK/NIDDK NIH HHS/United States
- R01 DK039773/DK/NIDDK NIH HHS/United States
- DK73628/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

