De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder
- PMID: 23979605
- PMCID: PMC4046646
- DOI: 10.1038/mp.2013.102
De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder
Abstract
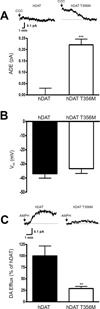
De novo genetic variation is an important class of risk factors for autism spectrum disorder (ASD). Recently, whole-exome sequencing of ASD families has identified a novel de novo missense mutation in the human dopamine (DA) transporter (hDAT) gene, which results in a Thr to Met substitution at site 356 (hDAT T356M). The dopamine transporter (DAT) is a presynaptic membrane protein that regulates dopaminergic tone in the central nervous system by mediating the high-affinity reuptake of synaptically released DA, making it a crucial regulator of DA homeostasis. Here, we report the first functional, structural and behavioral characterization of an ASD-associated de novo mutation in the hDAT. We demonstrate that the hDAT T356M displays anomalous function, characterized as a persistent reverse transport of DA (substrate efflux). Importantly, in the bacterial homolog leucine transporter, substitution of A289 (the homologous site to T356) with a Met promotes an outward-facing conformation upon substrate binding. In the substrate-bound state, an outward-facing transporter conformation is required for substrate efflux. In Drosophila melanogaster, the expression of hDAT T356M in DA neurons-lacking Drosophila DAT leads to hyperlocomotion, a trait associated with DA dysfunction and ASD. Taken together, our findings demonstrate that alterations in DA homeostasis, mediated by aberrant DAT function, may confer risk for ASD and related neuropsychiatric conditions.
Figures


Comment in
-
Drosophila melanogaster: a novel animal model for the behavioral characterization of autism-associated mutations in the dopamine transporter gene.Mol Psychiatry. 2013 Dec;18(12):1235. doi: 10.1038/mp.2013.157. Mol Psychiatry. 2013. PMID: 24253181 No abstract available.
References
-
- Devlin B, Scherer SW. Genetic architecture in autism spectrum disorder. Current opinion in genetics & development. 2012;22(3):229–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007628/GM/NIGMS NIH HHS/United States
- F31 DA 035535-01/DA/NIDA NIH HHS/United States
- R01 NS049261/NS/NINDS NIH HHS/United States
- DA13975/DA/NIDA NIH HHS/United States
- F31 DA035535/DA/NIDA NIH HHS/United States
- R01 DA013975/DA/NIDA NIH HHS/United States
- U54 HG003273/HG/NHGRI NIH HHS/United States
- U54 GM087519/GM/NIGMS NIH HHS/United States
- P50 HD055751/HD/NICHD NIH HHS/United States
- P01 DA012408/DA/NIDA NIH HHS/United States
- P01 DA12408/DA/NIDA NIH HHS/United States
- U54-GM087519/GM/NIGMS NIH HHS/United States
- MH089482/MH/NIMH NIH HHS/United States
- R01 MH089482/MH/NIMH NIH HHS/United States
- R56 DA013975/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

