Distinct signaling mechanisms regulate migration in unconfined versus confined spaces
- PMID: 23979717
- PMCID: PMC3760608
- DOI: 10.1083/jcb.201302132
Distinct signaling mechanisms regulate migration in unconfined versus confined spaces
Abstract
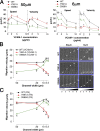
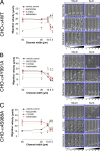
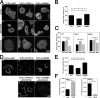
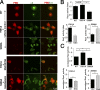
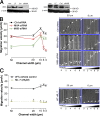
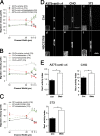
Using a microchannel assay, we demonstrate that cells adopt distinct signaling strategies to modulate cell migration in different physical microenvironments. We studied α4β1 integrin-mediated signaling, which regulates cell migration pertinent to embryonic development, leukocyte trafficking, and melanoma invasion. We show that α4β1 integrin promotes cell migration through both unconfined and confined spaces. However, unlike unconfined (2D) migration, which depends on enhanced Rac1 activity achieved by preventing α4/paxillin binding, confined migration requires myosin II-driven contractility, which is increased when Rac1 is inhibited by α4/paxillin binding. This Rac1-myosin II cross talk mechanism also controls migration of fibroblast-like cells lacking α4β1 integrin, in which Rac1 and myosin II modulate unconfined and confined migration, respectively. We further demonstrate the distinct roles of myosin II isoforms, MIIA and MIIB, which are primarily required for confined and unconfined migration, respectively. This work provides a paradigm for the plasticity of cells migrating through different physical microenvironments.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

