Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: evidence of stage migration from the ACCENT database
- PMID: 23980089
- PMCID: PMC3804289
- DOI: 10.1200/JCO.2013.49.4344
Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: evidence of stage migration from the ACCENT database
Abstract
Purpose: With improved patient care, better diagnosis, and more treatment options after tumor recurrence, outcomes after fluorouracil (FU) -based treatment are expected to have improved over time in early-stage colon cancer. Data from 18,449 patients enrolled onto 21 phase III trials conducted from 1978 to 2002 were evaluated for potential differences in time to recurrence (TTR), time from recurrence to death (TRD), and overall survival (OS) with regard to FU-based adjuvant regimens.
Methods: Trials were predefined as old versus newer era using initial accrual before or after 1995. Outcomes were compared between patients enrolled onto old- or newer-era trials, stratified by stage.
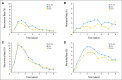
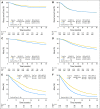
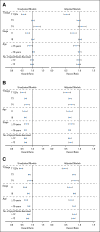
Results: Within the first 3 years, recurrence rates were lower in newer- versus old-era trials for patients with stage II disease, with no differences among those with stage III disease. Both TRD and OS were significantly longer in newer-era trials overall and within each stage. The lymph node (LN) ratio (ie, number of positive nodes divided by total nodes harvested) in those with stage III disease declined over time. TTR improved slightly, with larger number of LNs examined in both stages.
Conclusion: Improved TRD in newer trials supports the premise that more aggressive intervention (oxaliplatin- and irinotecan-based chemotherapy and/or surgery for recurrent disease) improves OS for patients previously treated in the adjuvant setting. Lower recurrence rates with identical treatments in those with stage II disease enrolled onto newer-era trials reflect stage migration over time, calling into question historical data related to the benefit of FU-based adjuvant therapy in such patients.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. - PubMed
-
- Petrelli N, Douglass HO, Jr, Herrera L, et al. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: A prospective randomized phase III trial—Gastrointestinal Tumor Study Group. J Clin Oncol. 1989;7:1419–1426. - PubMed
-
- Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer. Evidence in terms of response rate—Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1992;10:896–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

