Stomach cancer risk after treatment for hodgkin lymphoma
- PMID: 23980092
- PMCID: PMC3770865
- DOI: 10.1200/JCO.2013.50.6832
Stomach cancer risk after treatment for hodgkin lymphoma
Abstract
Purpose: Treatment-related stomach cancer is an important cause of morbidity and mortality among the growing number of Hodgkin lymphoma (HL) survivors, but risks associated with specific HL treatments are unclear.
Patients and methods: We conducted an international case-control study of stomach cancer nested in a cohort of 19,882 HL survivors diagnosed from 1953 to 2003, including 89 cases and 190 matched controls. For each patient, we quantified cumulative doses of specific alkylating agents (AAs) and reconstructed radiation dose to the stomach tumor location.
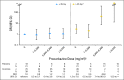
Results: Stomach cancer risk increased with increasing radiation dose to the stomach (Ptrend < .001) and with increasing number of AA-containing chemotherapy cycles (Ptrend = .02). Patients who received both radiation to the stomach ≥ 25 Gy and high-dose procarbazine (≥ 5,600 mg/m(2)) had strikingly elevated stomach cancer risk (25 cases, two controls; odds ratio [OR], 77.5; 95% CI, 14.7 to 1452) compared with those who received radiation < 25 Gy and procarbazine < 5,600 mg/m(2) (Pinteraction < .001). Risk was also elevated (OR, 2.8; 95% CI, 1.3 to 6.4) among patients who received radiation to the stomach ≥ 25 Gy but procarbazine < 5,600 mg/m(2); however, no procarbazine-related risk was evident with radiation < 25 Gy. Treatment with dacarbazine also increased stomach cancer risk (12 cases, nine controls; OR, 8.8; 95% CI, 2.1 to 46.6), after adjustment for radiation and procarbazine doses.
Conclusion: Patients with HL who received subdiaphragmatic radiotherapy had dose-dependent increased risk of stomach cancer, with marked risks for patients who also received chemotherapy containing high-dose procarbazine. For current patients, risks and benefits of exposure to both procarbazine and subdiaphragmatic radiotherapy should be weighed carefully. For patients treated previously, GI symptoms should be evaluated promptly.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009. http://seer.cancer.gov/csr/1975_2009_pops09/
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305:2311–2319. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

