Entering the era of nanoscience: time to be so small
- PMID: 23980495
- PMCID: PMC3768021
- DOI: 10.1166/jbn.2013.1642
Entering the era of nanoscience: time to be so small
Abstract
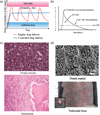
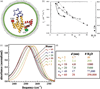
The field of nanoscience has produced more hype than probably any other branch of materials science and engineering in its history. Still, the potentials of this new field largely lay undiscovered ahead of us; what we have learnt so far with respect to the peculiarity of physical processes on the nanoscale is only the tip of an iceberg. Elaborated in this critical review is the idea that the surge of interest in physical chemistry of phenomena at the nanoscale presents a natural consequence of the spatial refinement of the human ability to controllably manipulate the substratum of our physical reality. Examples are given to illustrate the sensitivity of material properties to grain size on the nanoscale, a phenomenon that directly contributed to the rise of nanoscience as a special field of scientific inquiry. Main systemic challenges faced by the present and future scientists in this field are also mentioned. In part, this perspective article resembles standing on the constantly expanding seashore of the coast of nanoscience and nanoengineering and envisioning the parts of the island where the most significant advances may be expected to occur and where, therefore, most of the attention of scientist in this field is to be directed: (a) crossing the gap between life science and materials science; (b) increasing experimentation sensitivity; (c) crisscrossing theory and experiments; and (d) conjoining top-down and bottom-up synthetic approaches. As for materials and the application areas discussed, a special emphasis is placed on calcium phosphate nanoparticles and their usage in controlled drug delivery devices and other applications of biomedical relevance. It is argued that the properties of nanoparticles as drug carriers often comprise the critical determinant for- the efficacy of the drug therapy. Therefore, the basic properties of nanoparticles to be optimized for the purpose of maximizing this efficacy are discussed: size, size distribution, morphology, polymorphic nature, crystallinity, biocompatibility, biodegradability, drug elution profiles, and aggregation propensity.
Figures

















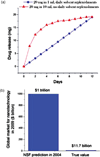
References
-
- Uskoković V. Nanomaterials and nanotechnologies: Approaching the crest of this big wave. Curr. Nanosci. 2008;4:119.
-
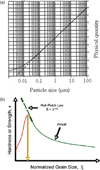
- Chokski AH, Rosen A, Karch J, Gleiter H. On the validity of the Hall-Petch relationship in nanocrystalline materials. Scripta Metall. 1989;23:1679.
-
- Andrievski RA. Fundamentals of nanostructured materials: Possibilities and problems. Moscow: BINOM; 2012.
-
- Pande CS, Cooper KP. Nanomechanics of Hall-Petch relationship in nanocrystalline materials. Progress Mat. Sci. 2009;54:689.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
