Validation of analytical methods in compliance with good manufacturing practice: a practical approach
- PMID: 23981284
- PMCID: PMC3765465
- DOI: 10.1186/1479-5876-11-197
Validation of analytical methods in compliance with good manufacturing practice: a practical approach
Abstract
Background: The quality and safety of cell therapy products must be maintained throughout their production and quality control cycle, ensuring their final use in the patient. We validated the Lymulus Amebocyte Lysate (LAL) test and immunophenotype according to International Conference on Harmonization Q2 Guidelines and the EU Pharmacopoeia, considering accuracy, precision, repeatability, linearity and range.
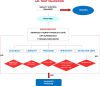
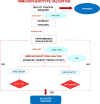
Methods: For the endotoxin test we used a kinetic chromogenic LAL test. As this is a limit test for the control of impurities, in compliance with International Conference on Harmonization Q2 Guidelines and the EU Pharmacopoeia, we evaluated the specificity and detection limit.For the immunophenotype test, an identity test, we evaluated specificity through the Fluorescence Minus One method and we repeated all experiments thrice to verify precision. The immunophenotype validation required a performance qualification of the flow cytometer using two types of standard beads which have to be used daily to check cytometer reproducibly set up. The results were compared together.Collected data were statistically analyzed calculating mean, standard deviation and coefficient of variation percentage (CV%).
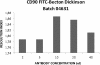
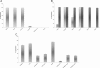
Results: The LAL test is repeatable and specific. The spike recovery value of each sample was between 0.25 EU/ml and 1 EU/ml with a CV% < 10%. The correlation coefficient (≥ 0.980) and CV% (< 10%) of the standard curve tested in duplicate showed the test's linearity and a minimum detectable concentration value of 0.005 EU/ml.The immunophenotype method performed thrice on our cell therapy products is specific and repeatable as showed by CV% inter -experiment < 10%.
Conclusions: Our data demonstrated that validated analytical procedures are suitable as quality controls for the batch release of cell therapy products.Our paper could offer an important contribution for the scientific community in the field of CTPs, above all to small Cell Factories such as ours, where it is not always possible to have CFR21 compliant software.
Figures


References
-
- EudraLex. The Rules Governing Medicinal Products in the European Union, Volume 4, Good Manufacturing Practice, Medicinal Products for Human and Veterinary Use. Ann Rev Mar Sci. 2001;15
-
- International conference on the harmonization of technical requirements for the registration of pharmaceuticals for human use. ICH harmonized tripartite guideline validation of analytical procedures: text and methodology Q2 (R1).Current Step 4 version, Parent Guideline dated 27 October 1994 (Complementary Guideline on Methodology dated 6 November 1996 incorporated in November. 2005.
-
- European P. European Directorate for the Quality of Medicines & HealthCare (EDQM) 7. 2011.
-
- S.W: Analytical Methods. A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods. BioPharm International. 2006.
-
- Gunetti M, Castiglia S, Rustichelli D, Mareschi K, Sanavio F, Muraro M, Signorino E, Castello L, Ferrero I, Fagioli F. Validation of analytical methods in GMP: the disposable Fast Read 102(R) device, an alternative practical approach for cell counting. J Transl Med. 2012;10:112. doi: 10.1186/1479-5876-10-112. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

