A novel serogenetic approach determines the community prevalence of celiac disease and informs improved diagnostic pathways
- PMID: 23981538
- PMCID: PMC3765645
- DOI: 10.1186/1741-7015-11-188
A novel serogenetic approach determines the community prevalence of celiac disease and informs improved diagnostic pathways
Abstract
Background: Changing perspectives on the natural history of celiac disease (CD), new serology and genetic tests, and amended histological criteria for diagnosis cast doubt on past prevalence estimates for CD. We set out to establish a more accurate prevalence estimate for CD using a novel serogenetic approach.
Methods: The human leukocyte antigen (HLA)-DQ genotype was determined in 356 patients with 'biopsy-confirmed' CD, and in two age-stratified, randomly selected community cohorts of 1,390 women and 1,158 men. Sera were screened for CD-specific serology.
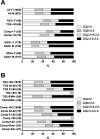
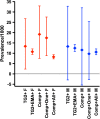
Results: Only five 'biopsy-confirmed' patients with CD did not possess the susceptibility alleles HLA-DQ2.5, DQ8, or DQ2.2, and four of these were misdiagnoses. HLA-DQ2.5, DQ8, or DQ2.2 was present in 56% of all women and men in the community cohorts. Transglutaminase (TG)-2 IgA and composite TG2/deamidated gliadin peptide (DGP) IgA/IgG were abnormal in 4.6% and 5.6%, respectively, of the community women and 6.9% and 6.9%, respectively, of the community men, but in the screen-positive group, only 71% and 75%, respectively, of women and 65% and 63%, respectively, of men possessed HLA-DQ2.5, DQ8, or DQ2.2. Medical review was possible for 41% of seropositive women and 50% of seropositive men, and led to biopsy-confirmed CD in 10 women (0.7%) and 6 men (0.5%), but based on relative risk for HLA-DQ2.5, DQ8, or DQ2.2 in all TG2 IgA or TG2/DGP IgA/IgG screen-positive subjects, CD affected 1.3% or 1.9%, respectively, of females and 1.3% or 1.2%, respectively, of men. Serogenetic data from these community cohorts indicated that testing screen positives for HLA-DQ, or carrying out HLA-DQ and further serology, could have reduced unnecessary gastroscopies due to false-positive serology by at least 40% and by over 70%, respectively.
Conclusions: Screening with TG2 IgA serology and requiring biopsy confirmation caused the community prevalence of CD to be substantially underestimated. Testing for HLA-DQ genes and confirmatory serology could reduce the numbers of unnecessary gastroscopies.
Figures
References
-
- Biagi F, Klersy C, Balduzzi D, Corazza GR. Are we not over-estimating the prevalence of coeliac disease in the general population? Ann Med. 2010;42:557–561. - PubMed
-
- Husby S, Koletzko S, Korponay-Szabo IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C. et al.European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. - PubMed
-
- National Institute for Health and Clinicial Excellence. NICE Guidelines. 2009. http://www.nice.org.uk/CG86.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

