Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis
- PMID: 23981726
- PMCID: PMC3785263
- DOI: 10.5966/sctm.2013-0032
Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis
Abstract
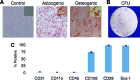
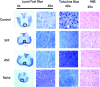
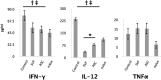
Administration of adipose-derived stromal/stem cells (ASCs) represents a promising therapeutic approach for autoimmune diseases since they have been shown to have immunomodulatory properties. The uncultured, nonexpanded counterpart of ASCs, the stromal vascular fraction (SVF), is composed of a heterogeneous mixture of cells. Although administration of ex vivo culture-expanded ASCs has been used to study immunomodulatory mechanisms in multiple models of autoimmune diseases, less is known about SVF-based therapy. The ability of murine SVF cells to treat myelin oligodendrocyte glycoprotein35-55-induced experimental autoimmune encephalitis (EAE) was compared with that of culture-expanded ASCs in C57Bl/6J mice. A total of 1 × 10(6) SVF cells or ASCs were administered intraperitoneally concomitantly with the induction of disease. The data indicate that intraperitoneal administration of ASCs significantly ameliorated the severity of disease course. They also demonstrate, for the first time, that the SVF effectively inhibited disease severity and was statistically more effective than ASCs. Both cell therapies also demonstrated a reduction in tissue damage, a decrease in inflammatory infiltrates, and a reduction in sera levels of interferon-γ and interleukin-12. Based on these data, SVF cells effectively inhibited EAE disease progression more than culture-expanded ASCs.
Keywords: Adipose; Adult stem cells; Neuroimmune; Stem cells; Tissue-specific stem cells.
Figures

References
-
- The MS disease-modifying medications. [Accessed April 11, 2012]. Available at http://www.nationalmssociety.org.
-
- Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. - PubMed
-
- Peinado JR, Pardo M, de la Rosa O, et al. Proteomic characterization of adipose tissue constituents, a necessary step for understanding adipose tissue complexity. Proteomics. 2012;12:607–620. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

