Efficacy of omega-3 for vasomotor symptoms treatment: a randomized controlled trial
- PMID: 23982113
- PMCID: PMC4072122
- DOI: 10.1097/GME.0b013e31829e40b8
Efficacy of omega-3 for vasomotor symptoms treatment: a randomized controlled trial
Abstract
Objective: This study aims to determine the efficacy and tolerability of omega-3 fatty acids in reducing vasomotor symptoms (VMS) frequency and bother in perimenopausal and postmenopausal women.
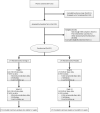
Methods: This study was a 12-week, three-by-two factorial, randomized controlled trial. Eligible women were randomized to a double-blind comparison of omega-3 (n = 177) or placebo (n = 178) capsules, and simultaneously to yoga (n = 107), aerobic exercise (n = 106), or their usual physical activity (n = 142). Participants received 1.8 g of omega-3 daily for 12 weeks. Each capsule contained ethyl eicosapentaenoic acid (425 mg), docosahexaenoic acid (100 mg), and other omega-3s (90 mg). Primary outcomes were VMS frequency and bother. Secondary outcomes included sleep quality (Pittsburgh Sleep Quality Index), insomnia symptoms (Insomnia Severity Index), depressive symptoms (Physician's Health Questionnaire-8), and anxiety (Generalized Anxiety Disorder-7).
Results: The mean baseline frequency of VMS per day was 7.6 (95% CI, 7.0 to 8.2). After 12 weeks, the reduction in VMS frequency with omega-3 (-2.5; 95% CI, -3.0 to -1.9) did not differ significantly from that with placebo (-2.7; 95% CI, -3.3 to -2.2), with a relative difference of 0.3 fewer hot flashes per day (95% CI, -0.5 to 1.0; P = 0.28). Changes in VMS bother at 12 weeks were also similar between groups, with no relative difference on a four-point scale (95% CI, -0.1 to 0.2; P = 0.36). Omega-3s compared with placebo showed no improvement in self-reported sleep or mood (P > 0.09 for all comparisons).
Conclusions: Among healthy, sedentary perimenopausal and postmenopausal women, a 12-week treatment with omega-3 does not improve VMS frequency, VMS bother, sleep, or mood compared with placebo.
Trial registration: ClinicalTrials.gov NCT01178892.
Figures

Comment in
-
Menopause strategies: Finding Lasting Answers for Symptoms and Health: eliminating hot flashes--still not a slam dunk!Menopause. 2014 Apr;21(4):321-2. doi: 10.1097/GME.0000000000000217. Menopause. 2014. PMID: 24552975 No abstract available.
-
Three alternative ways to treat VMS failed.Climacteric. 2014 Aug;17(4):512-3. Climacteric. 2014. PMID: 25147889 No abstract available.
References
-
- Freeman EW, Sammel MD, Lin H, Gracia CR, Pien GW, Nelson DB, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007 Aug;110(2 Pt 1):230–40. - PubMed
-
- Hirschfeld RM. Long-term side effects of SSRIs: sexual dysfunction and weight gain. J Clin Psychiatry. 2003;64(Suppl 18):20–4. - PubMed
-
- Barnes PMBB, Nahin R. CDC National Health Statistics Report #12. Complementary and Alternative Medicine Use Among Adults and Children: United States, 2007. 2008. - PubMed
-
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998 Nov 11;280(18):1569–75. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01AG032659/AG/NIA NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 AG032682/AG/NIA NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- U01 AG032659/AG/NIA NIH HHS/United States
- U01AG032699/AG/NIA NIH HHS/United States
- U01AG032700/AG/NIA NIH HHS/United States
- U01 AG032699/AG/NIA NIH HHS/United States
- U01AG032669/AG/NIA NIH HHS/United States
- U01 AG032656/AG/NIA NIH HHS/United States
- U01 AG032700/AG/NIA NIH HHS/United States
- U01AG032682/AG/NIA NIH HHS/United States
- UL1RR02571/RR/NCRR NIH HHS/United States
- U01 AG032669/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

