Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury
- PMID: 23982144
- PMCID: PMC3834784
- DOI: 10.1096/fj.13-236380
Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury
Abstract
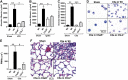
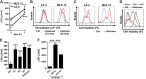
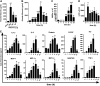
We investigated how complement activation promotes tissue injury and organ dysfunction during acute inflammation. Three models of acute lung injury (ALI) induced by LPS, IgG immune complexes, or C5a were used in C57BL/6 mice, all models requiring availability of both C5a receptors (C5aR and C5L2) for full development of ALI. Ligation of C5aR and C5L2 with C5a triggered the appearance of histones (H3 and H4) in bronchoalveolar lavage fluid (BALF). BALF from humans with ALI contained H4 histone. Histones were absent in control BALF from healthy volunteers. In mice with ALI, in vivo neutralization of H4 with IgG antibody reduced the intensity of ALI. Neutrophil depletion in mice with ALI markedly reduced H4 presence in BALF and was highly protective. The direct lung damaging effects of extracellular histones were demonstrated by airway administration of histones into mice and rats (Sprague-Dawley), which resulted in ALI that was C5a receptor-independent, and associated with intense inflammation, PMN accumulation, damage/destruction of alveolar epithelial cells, together with release into lung of cytokines/chemokines. High-resolution magnetic resonance imaging demonstrated lung damage, edema and consolidation in histone-injured lungs. These studies confirm the destructive C5a-dependent effects in lung linked to appearance of extracellular histones.
Keywords: complement; cytotoxic factors.
Figures


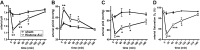


References
-
- Goss C. H., Brower R. G., Hudson L. D., Rubenfeld G. D. (2003) Incidence of acute lung injury in the United States. Crit. Care Med. 31, 1607–1611 - PubMed
-
- Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) Incidence and outcomes of acute lung injury. N Engl. J. Med. 353, 1685–1693 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

