Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial
- PMID: 23982301
- PMCID: PMC3992936
- DOI: 10.1176/appi.ajp.2013.13030392
Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial
Abstract
Objective: Ketamine, a glutamate N-methyl-d-aspartate (NMDA) receptor antagonist, has shown rapid antidepressant effects, but small study groups and inadequate control conditions in prior studies have precluded a definitive conclusion. The authors evaluated the rapid antidepressant efficacy of ketamine in a large group of patients with treatment-resistant major depression.
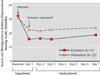
Method: This was a two-site, parallel-arm, randomized controlled trial of a single infusion of ketamine compared to an active placebo control condition, the anesthetic midazolam. Patients with treatment-resistant major depression experiencing a major depressive episode were randomly assigned under double-blind conditions to receive a single intravenous infusion of ketamine or midazolam in a 2:1 ratio (N=73). The primary outcome was change in depression severity 24 hours after drug administration, as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS).
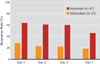
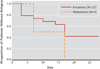
Results: The ketamine group had greater improvement in the MADRS score than the midazolam group 24 hours after treatment. After adjustment for baseline scores and site, the MADRS score was lower in the ketamine group than in the midazolam group by 7.95 points (95% confidence interval [CI], 3.20 to 12.71). The likelihood of response at 24 hours was greater with ketamine than with midazolam (odds ratio, 2.18; 95% CI, 1.21 to 4.14), with response rates of 64% and 28%, respectively.
Conclusions: Ketamine demonstrated rapid antidepressant effects in an optimized study design, further supporting NMDA receptor modulation as a novel mechanism for accelerated improvement in severe and chronic forms of depression. More information on response durability and safety is required before implementation in clinical practice.
Trial registration: ClinicalTrials.gov NCT00768430.
Figures
Comment in
-
Ketamine for treatment-resistant depression: ready or not for clinical use?Am J Psychiatry. 2013 Oct;170(10):1079-81. doi: 10.1176/appi.ajp.2013.13081034. Am J Psychiatry. 2013. PMID: 23982324 No abstract available.
-
A word to the wise about ketamine.Am J Psychiatry. 2014 Mar;171(3):262-4. doi: 10.1176/appi.ajp.2014.13101434. Am J Psychiatry. 2014. PMID: 24585328 No abstract available.
References
-
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Anderson W, Dhansay MA, Phillips A, Shurin S, Walport M, Ewart W, Savill SJ, Bordin IA, Costello EJ, Durkin M, Fairburn C, Glass RI, Hall W, Huang Y, Hyman SE, Jamison K, Kaaya S, Kapur S, Kleinman A, Ogunniyi A, Otero-Ojeda A, Poo MM, Ravindranath V, Sahakian BJ, Saxena S, Singer PA, Stein DJ. Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health: Grand challenges in global mental health. Nature. 2011;475:27–30. - PMC - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M. STAR*D Study Team: Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–1242. - PubMed
-
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ. STAR*D Study Team: Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

