OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation
- PMID: 23982340
- PMCID: PMC3864174
- DOI: 10.1097/TP.0b013e3182a203bd
OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation
Erratum in
- Transplantation. 2014 Mar 27;97(6):38
Abstract
Background: The once-daily (QD), prolonged-release formulation of tacrolimus has been shown to improve adherence versus twice-daily (BD) tacrolimus. Treatment nonadherence in transplant recipients has been associated with poor graft outcomes.
Methods: This open-label, parallel-group study randomized adults with end-stage renal disease undergoing primary kidney transplantation or retransplantation to an initial dose of tacrolimus BD 0.2 mg/kg per day (Arm 1; n=309), QD 0.2 mg/kg per day (Arm 2; n=302), QD 0.3 mg/kg per day (Arm 3; n=304) all with mycophenolate mofetil and corticosteroids (tapered) over 24 weeks, or tacrolimus QD 0.2 mg/kg per day with mycophenolate mofetil, basiliximab, and corticosteroids given only perioperatively (Arm 4; n=283). The primary composite endpoint (efficacy failure; per protocol set) was defined as graft loss, biopsy-confirmed acute rejection, or graft dysfunction at week 24. Graft dysfunction was defined as estimated glomerular filtration rate Modification of Diet in Renal Disease-4 formula of less than 40 mL/min/1.73 m(2). The prespecified noninferiority margin was 12.5%.
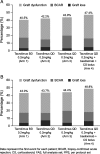
Results: The per protocol set included 976 patients: 237, 263, 246, and 230 patients in Arms 1 to 4, respectively. Noninferiority of the composite endpoint was demonstrated for Arm 2 versus Arm 1; Kaplan-Meier estimates of efficacy failure were 42.2% and 40.6%, respectively (difference, -1.6%; 95% confidence interval [CI], -12.2% to 9.0%). Noninferiority to Arm 1 was not confirmed for Arm 3 (difference, -3.5%; 95% CI, -13.6% to 6.6%) or Arm 4 (difference, -7.1%; 95% CI, -16.1% to 1.9%). Graft dysfunction (estimated glomerular filtration rate <40 mL/min/1.73 m(2)) was the main determinant of composite-endpoint efficacy failure across all arms.
Conclusions: In patients representative of the European kidney transplant population, tacrolimus QD-based immunosuppression (0.2 mg/kg/day), without induction, showed similar efficacy to 0.2 mg/kg per day tacrolimus BD.
Trial registration: ClinicalTrials.gov NCT00717470.
Figures


References
-
- European Medicines Agency. Committee for Medicinal Products for Human Use (2012). CHMP Pharmacokinetics Working Party (PKWP). Questions and answers: positions on specific questions addressed to the Pharmacokinetics Working Party. London. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed May 2012.
-
- Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol 2007; 2: 374. - PubMed
-
- Wu MJ, Cheng CY, Chen CH, et al. Lower variability of tacrolimus trough concentration after conversion from Prograf to Advagraf in stable kidney transplant recipients. Transplantation 2011; 92: 648. - PubMed
-
- Borra LC, Roodnat JI, Kal JA, et al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010; 25: 2757. - PubMed
-
- Stevenson KS, Glen J, Stevens KK, et al. High tacrolimus intrapatient variability is associated with acute rejection and graft loss (abstract MO-021). Transpl Int 2011. (Suppl 2); 24: 111.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

