Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization
- PMID: 23982586
- PMCID: PMC3755284
- DOI: 10.1038/srep02534
Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization
Erratum in
- Sci Rep. 2014;4:5138
Abstract
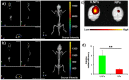
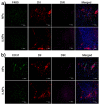
Nanoparticles (NPs) were widely used in drugs/probes delivery for improved disease diagnosis and/or treatment. Targeted delivery to cancer cells is a highly attractive application of NPs. However, few studies have been performed on the targeting mechanisms of these ligand-modified delivery systems. Additional studies are needed to understand the transport of nanoparticles in the cancer site, the interactions between nanoparticles and cancer cells, the intracellular trafficking of nanoparticles within the cancer cells and the subcellular destiny and potential toxicity. Interleukin 13 (IL-13) peptide can specifically bind IL-13Rα2, a receptor that is highly expressed on glioma cells but is expressed at low levels on other normal cells. It was shown that the nanoparticels modification with the IL-13 peptide could improve glioma treatment by selectively increasing cellular uptake, facilitating cell internalization, altering the uptake pathway and increasing glioma localization.
Figures





References
-
- Matsumura Y. & Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46, 6387–92 (1986). - PubMed
-
- Jain R. K. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev 46, 149–68 (2001). - PubMed
-
- Mickler F. M. et al. Tuning Nanoparticle Uptake: Live-Cell Imaging Reveals Two Distinct Endocytosis Mechanisms Mediated by Natural and Artificial EGFR Targeting Ligand. Nano Lett 12, 3417–23 (2012). - PubMed
-
- Liu Y. & Lu W. Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin Drug Deliv 9, 671–86 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

