Haloperidol dose for the acute phase of schizophrenia
- PMID: 23983042
- PMCID: PMC11622733
- DOI: 10.1002/14651858.CD001951.pub2
Haloperidol dose for the acute phase of schizophrenia
Abstract
Background: Haloperidol is a benchmark, accessible antipsychotic drug against which the effects of newer treatments are gauged.
Objectives: To determine the best range of doses for haloperidol for the treatment of people acutely ill with schizophrenia.
Search methods: We searched the Cochrane Schizophrenia Group Trials Register (February 2010), which is based on regular searches of CINAHL, EMBASE, MEDLINE and PsycINFO.
Selection criteria: We selected studies if they involved people being treated for acute schizophrenia, randomised to two or more dose ranges of non-depot haloperidol, and if they reported clinically meaningful outcomes.
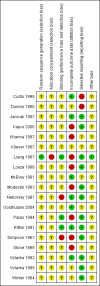
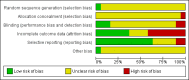
Data collection and analysis: For this update, we inspected all citations and independently re-inspected a sample of citations in order to ensure reliable selection. We resolved any disagreement by discussion, and where doubt remained, we acquired the full-text article for further inspection. We then ordered papers, and reliably re-inspected and quality assessed the full reports, and extracted data. For homogeneous dichotomous data, we calculated the risk ratio (RR) with 95% confidence intervals (CI) on an intention-to-treat (ITT) basis. We assumed that people who left the study early or were lost to follow-up had a negative outcome. We calculated mean differences (MD) for continuous outcomes that reported ITT, last observation carried forward (LOCF) data. We excluded data if loss to follow-up was greater than 50%.
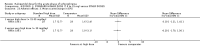
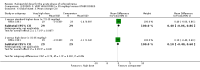
Main results: We included 19 trials with 19 different randomised dose comparisons. No studies reported data on relapse rates or quality of life and only one compared low dose (> 1.5 to 3 mg/day) haloperidol to higher dose ranges. Using standard lower dose (> 3 to 7.5 mg/day) did not result in loss of efficacy (no clinically important improvement in global state, versus standard higher dose (> 7.5 to 15 mg/day, n = 48, 1 RCT, RR 1.09, 95% CI 0.7 to 1.8, very-low-quality evidence); versus high dose (> 15 to 35 mg/day, n = 81, 2 RCTs, RR 0.95, 95% CI 0.8 to 1.2, very-low-quality evidence). Doses of haloperidol in the range of > 3 to 7.5 mg/day had a lower rate of development of clinically significant extrapyramidal adverse effects than higher doses (clinically significant extrapyramidal adverse effects, versus standard higher dose, n = 64, 2 RCTs, RR 0.12, 95% CI 0.01 to 2.1, very-low-quality evidence); versus high dose, n = 144, 3 RCTs, RR 0.59, 95% CI 0.5 to 0.8, very-low-quality evidence; versus very high dose (> 35 mg/day, n = 86, 2 RCTs, RR 0.70, 95% CI 0.5 to 1.1, very-low-quality evidence). None of the other comparisons between dose ranges yielded statistically significant differences, but several, particularly with lower dose ranges, were underpowered to detect clinically meaningful differences.
Authors' conclusions: Noresults were conclusive and all were based on small, short studies of limited quality. However, it would be understandable if clinicians were cautious in prescribing doses in excess of 7.5 mg/day of haloperidol to a person with uncomplicated acute schizophrenia, and if people with schizophrenia were equally reticent to take greater doses. Further research is needed regarding the efficacy and tolerability of the lower dose ranges, especially > 1.5 to 3 mg/day.
Conflict of interest statement
None known.
Figures




























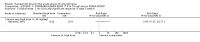

































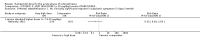

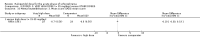



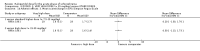


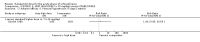












Update of
-
Haloperidol dose for the acute phase of schizophrenia.Cochrane Database Syst Rev. 2002;(3):CD001951. doi: 10.1002/14651858.CD001951. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2013 Aug 28;(8):CD001951. doi: 10.1002/14651858.CD001951.pub2. PMID: 12137638 Updated.
References
References to studies included in this review
Curtis 1995 {published data only}
-
- Curtis CE, Mann M, Piscani K, Burr G, Hitzemann RJ, Hirschowitz J. A neuroendocrine method of antipsychotic dose reduction in schizophrenia. Proceedings of the 148th Annual Meeting of the American Psychiatric Association; 1995 May 20‐25; Miami. 1995.
Donlon 1980 {published data only}
-
- Donlon PT, Hopkin JT, Tupin JP, Wicks JJ, Wahba M, Meadow A. Haloperidol for acute schizophrenic patients. An evaluation of three oral regimens. Archives of General Psychiatry 1980;6:691‐5. - PubMed
-
- Wahba M, Donlon PT, Meadow A. Cognitive changes in acute schizophrenia with brief neuroleptic treatment. American Journal of Psychiatry 1981;138(10):1307‐10. - PubMed
Janicak 1997 {published data only}
-
- Janicak PG, Javaid JI, Sharma RP, Leach A, Dowd S, Davis JM. A two‐phase, double‐blind randomised study of three haloperidol plasma levels for acute psychosis with reassignment of initial non‐responders. Acta Psychiatrica Scandinavica 1997;4:343‐50. - PubMed
-
- Javaid JI, Janicak PG, Hedeker D, Sharma RP, Davis JM. Steady‐state plasma level prediction for haloperidol from a single test dose. Psychopharmacology Bulletin 1991;27(1):83‐8. - PubMed
-
- Javaid JI, Janicak PG, Sharma RP, Leach AM, Davis JM, Wang Z. Prediction of haloperidol steady‐state levels in plasma after a single test dose. Journal of Clinical Psychopharmacology 1996;16(1):45‐50. - PubMed
Kapur 2000 {published data only}
-
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double‐blind PET study of first‐episode schizophrenia. American Journal of Psychiatry 2000;4:514‐20. - PubMed
Khanna 1997 {published data only}
Klieser 1987 {published data only}
-
- Klieser E, Lehmann E. Experimental comparison of the effectivity of individually adapted and standardized dosages of haloperidol. Neuropsychobiology 1987;18(3):122‐6. - PubMed
-
- Klieser E, Lehmann E. Experimental comparison of the efficacy of standardized haloperidol treatment and adequate individual dosages in acute schizophrenic states. Fortschritte der Neurologie Psychiatrie 1992;3:126‐9. - PubMed
Liang 1987 {published data only}
-
- Liang S. Comparison of therapeutic effects between haloperidol and insulin coma for schizophrenia and the optimal blood level of haloperidol. Chinese Journal of Neurology and Psychiatry 1987;20(1):43‐8. - PubMed
Louza 1988 {published data only}
-
- Louza Neto MR, Muller Spahn F, Ruther E, Scherer J. Haloperidol plasma level after a test dose as predictor for the clinical response to treatment in acute schizophrenic patients. Pharmacopsychiatry 1988;21(5):226‐31. [MEDLINE: ] - PubMed
McEvoy 1991 {published data only}
-
- McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia. A controlled study of the neuroleptic threshold. Archives of General Psychiatry 1991;48(8):739‐45. - PubMed
-
- McEvoy JP, Schooler NR, Wilson WH. Predictors of therapeutic response to haloperidol in acute schizophrenia. Psychopharmacology Bulletin 1991;27(2):97‐101. - PubMed
Modestin 1983 {published data only}
-
- Modestin J, Toffler G, Pia M, Greub E. Haloperidol in acute schizophrenic inpatients. A double‐blind comparison of two dosage regimens. Pharmacopsychiatria 1983;16:4121‐6. - PubMed
Neborsky 1981 {published data only}
-
- Neborsky R, Depry D, Janowsky DS, Munson E, Perel J. Haloperidol plasma red blood‐cell ratios and clinical efficacy. Psychopharmacology Bulletin 1982;18(4):17‐20. - PubMed
-
- Neborsky R, Janowsky D, Munson E, Depry D. Rapid treatment of acute psychotic symptoms with high‐and low‐dose haloperidol. Behavioral considerations. Archives of General Psychiatry 1981;38(2):195‐9. - PubMed
-
- Neborsky R, Janowsky D, Munson E, Hornbeck C, Depry D. Behavioral prediction of response to haloperidol: a test dose strategy. Journal of Clinical Psychiatry 1982;43(4):157‐8. - PubMed
-
- Neborsky RJ, Janowsky DS, Perel JM. Red‐blood‐cell plasma haloperidol ratios and antipsychotic efficacy. Psychiatry Research 1982;6(1):123‐4. - PubMed
-
- Neborsky RJ, Janowsky DS, Perel JM, Munson E, Depry D. Plasma/RBC haloperidol ratios and improvement in acute psychotic symptoms. Journal of Clinical Psychiatry 1984;45(1):10‐3. - PubMed
Oosthuizen 2004 {published data only}
-
- Oosthuizen P, Emsley R, Turner HJ, Keyter N. A randomised, controlled comparison of the efficacy and tolerability of low and high doses of haloperidol in the treatment of first‐episode psychosis. International Journal of Neuropsychopharmacology 2004;7(2):125‐31. [MEDLINE: ] - PubMed
Palao 1994 {published data only}
-
- Palao DJ, Arauxo A, Brunet M, Bernardo M, Haro JM, Ferrer J, et al. Haloperidol: therapeutic window in schizophrenia. Journal of Clinical Psychopharmacology 1994;14(5):303‐10. - PubMed
-
- Palao DJ, Arauxo A, Brunet M, Marquez M, Bernardo M, Ferrer J, et al. Positive versus negative symptoms in schizophrenia: response to haloperidol. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 1994;18(1):155‐64. - PubMed
-
- Palao DJ, Arauxo A, Haro JM, Brunet M, Bernardo M, Volavka J, et al. The relationship between plasma haloperidol concentrations and clinical results. Archives of General Psychiatry 1996;53(12):1167‐9. - PubMed
Rifkin 1991 {published data only}
-
- Doddi S, Rifkin A, Karajgi B, Cooper T, Borenstein M. Blood levels of haloperidol and clinical outcome in schizophrenia. Journal of Clinical Psychopharmacology 1994;14(3):187‐95. - PubMed
-
- Rifkin A, Doddi S, Karajgi B, Borenstein M, Wachspress M. Dosage of haloperidol for schizophrenia. Archives of General Psychiatry 1991;48(2):166‐70. - PubMed
Simpson 1967 {published data only}
-
- Simpson GM, Angus JWS, Edwards JG. A controlled study of haloperidol in chronic schizophrenia. Current Therapeutic Research 1967;9(8):401‐12. - PubMed
Stone 1995 {published data only}
-
- Garver DL, Steinberg JL, McDermott BE, Yao JK, Ramberg JE, Lewis S, et al. Etiologic heterogeneity of the psychoses: is there a dopamine psychosis?. Neuropsychopharmacology 1997;16(3):191‐201. - PubMed
-
- Stone CK, Garver DL, Griffith J, Hirschowitz J, Bennett J. Further evidence of a dose‐response threshold for haloperidol in psychosis. American Journal of Psychiatry 1995;152(8):1210‐12. - PubMed
Volavka 1992 {published data only}
-
- Bitter I, Volavka J, Cooper J, Scheurer, Camus L, Bakall R. Haloperidol blood levels and clinical effects in schizophrenia. Proceedings of the 8th World Congress of Psychiatry; 1989 Oct 13‐19; Athens, Greece. 1989.
-
- Chou JC, Douyon R, Czobor P, Volavka J, Cooper TB. Change in plasma prolactin and clinical response to haloperidol in schizophrenia and schizoaffective disorder. Psychiatry Research 1998;81(1):51‐5. - PubMed
-
- Convit A, Volavka J, Czobor P, Asis J, Evangelista C. Effect of subtle neurological dysfunction on response to haloperidol treatment in schizophrenia. American Journal of Psychiatry 1994;151(1):49‐56. - PubMed
-
- Cooper TB, Volavka J, Czobor P. Plasma drug level and clinical response. Journal of Clinical Psychopharmacology 1992;12(2):134‐6. - PubMed
-
- Czobor P, Volavka J. Dimensions of the brief psychiatric rating scale: an examination of stability during haloperidol treatment. Comprehensive Psychiatry 1996;37(3):205‐15. - PubMed
Volavka 1995 {published data only}
-
- Chou JC, Douyon R, Czobor P, Volavka J, Cooper TB. Change in plasma prolactin and clinical response to haloperidol in schizophrenia and schizoaffective disorder. Psychiatry Research 1998;81(1):51‐5. - PubMed
-
- Czobor P, Volavka J. Dimensions of the Brief Psychiatric Rating Scale: an examination of stability during haloperidol treatment. Comprehensive Psychiatry 1996;37(3):205‐15. - PubMed
-
- Czobor P, Volavka J. Positive and negative symptoms: is their change related?. Schizophrenia Bulletin 1996;22(4):577‐90. - PubMed
-
- Volavka J, Cooper TB, Czobor P, Meisner M. Effect of varying haloperidol plasma levels on negative symptoms in schizophrenia and schizoaffective disorder. Psychopharmacology Bulletin 1996;32(1):75‐9. - PubMed
-
- Volavka J, Cooper TB, Czobor P, Meisner M. Haloperidol plasma levels and clinical effects. Psychopharmacology Bulletin 1995;31:536. - PubMed
Winter 1984 {published data only}
-
- Lehmann E, Lienert GA. Differential improvements from haloperidol in two types of schizophrenics. Psychopharmacology 1984;84(1):96‐7. - PubMed
-
- Winter M, Lehmann E, Scholz OB. Effects of high and low dosage of haloperidol on the brain in relation to schizophrenic thought disorder. Neuropsychobiology 1984;2‐3:115‐21. - PubMed
References to studies excluded from this review
Bjorndal 1980 {published data only}
-
- Bjorndal N, Bjerre M, Gerlach J, Kristjansen P, Magelund G, Oestrich IH, et al. High dosage haloperidol therapy in chronic schizophrenic patients: a double‐blind study of clinical response, side effects, serum haloperidol, and serum prolactin. Psychopharmacology 1980;67(1):17‐23. - PubMed
Boyer 1987 {published data only}
-
- Boyer P, Puech AJ. Determinants for clinical activity of neuroleptic drugs: chemical substances, doses, assessment tools [Modalities d'action clinique ses neuroleptiques: substances, doses, instruments de mesure utilises]. Psychiatrie and Psychobiologie 1987;2(4):296‐305.
Coryell 1990 {published data only}
-
- Coryell W, Kelly M, Perry PJ, Miller DD. Haloperidol plasma levels and acute clinical change in schizophrenia. Journal of Clinical Psychopharmacology 1990;10(6):397‐402. - PubMed
-
- Kelly MW, Perry PJ, Coryell WH, Miller DD, Arndt SV. Reduced haloperidol plasma‐concentration and clinical‐response in acute exacerbations of schizophrenia. Psychopharmacology 1990;102(4):514‐20. - PubMed
Coryell 1998 {published data only}
-
- Coryell W, Miller DD, Perry PJ. Haloperidol plasma levels and dose optimization. American Journal of Psychiatry 1998;155(1):48‐53. - PubMed
-
- Coryell WH, Miller DD, Perry PJ. The clinical utility of haloperidol plasma levels. Schizophrenia Research 1995;15(1‐2):146.
Davis 1985 {published data only}
-
- Davis JM, Ericksen SE, Hurt S, Chang SS, Javaid JI, Dekirmenjian H, et al. Blood levels of haloperidol and clinical response. Psychopharmacology Bulletin 1985;21(1):48‐51. - PubMed
-
- Ericksen SE, Hurt SW, Chang S. Haloperidol dose, plasma levels, and clinical response: a double‐blind study. Psychopharmacology Bulletin 1978;14(2):15‐6. - PubMed
-
- Hurt SW, Holzma PS, Davis JM. Thought disorder. The measurement of its changes. Archives of General Psychiatry 1983;40:1281‐5. - PubMed
Dubin 1985 {published data only}
-
- Dubin WR, Waxman HM, Weiss KJ, Ramchandani D, Tavani‐Petrone C. Rapid tranquilization: the efficacy of oral concentrate. Journal of Clinical Psychiatry 1985;46(11):475‐8. - PubMed
Dutoit 1995 {published and unpublished data}
-
- Dutoit D, Thomas P, Leroux JM, Vaiga G, Pommery J, Cottencin O, et al. Erythrocyte ketone reductase activity, total plasma haloperidol and acute psychoses [Activite cetone reductase erythrocytaire, taux plasmatiques d'haloperidol et psychoses aigues]. Encephale 1995;21(6):417‐24. - PubMed
Garver 1984 {published data only}
-
- Garver DL, Kelly K, Fried KA, Magnusson M, Hirschowitz J. Drug response patterns as a basis of nosology for the mood‐incongruent psychoses (the schizophrenias). Psychological Medicine 1988;18:873‐85. - PubMed
-
- Mavroidis ML, Garver DL, Kanter DR, Hirschowitz J. Plasma haloperidol levels and clinical response: confounding variables. Psychopharmacology Bulletin 1985;21(1):62‐5. [MEDLINE: ] - PubMed
-
- Mavroidis ML, Kanter DR, Hirschowitz J, Garver DL. Clinical response and plasma haloperidol levels in schizophrenia. Psychopharmacology 1983;81(4):354‐6. - PubMed
Garver 1985 {published data only}
-
- Garver DL, Hirschowitz J, Glicksteen GA, Kanter DR, Mavroidis ML. Haloperidol plasma and red blood cell levels and clinical antipsychotic response. Journal of Clinical Psychopharmacology 1984;4(3):133‐7. - PubMed
Gerlach 1985a {published data only}
-
- Gerlach J, Behnke K, Heltberg J, Munk‐Andersen, Nielsen H. Dogmatil and haloperidol for the treatment of schizophrenia. Double blind cross‐over study of therapeutic effectiveness, side effects and plasma concentrations [Le dogmatil et l'haloperidol dans le traitement de la schizophrenie. Etude croisee en double aveugle de l'action therapeutique, des effets secondaires et des concentrations plasmatiques]. Semaine des Hopitaux 1985;61(19):1309‐16.
Hirschowitz 1997 {published data only}
-
- Hirschowitz J, Hitzemann R, Piscani K, Burr G, Frecska E, Culliton D, et al. The Dose Reduction in Schizophrenia (DORIS) Study: a final report. Schizophrenia Research 1997;23(1):31‐43. - PubMed
Levin 1996 {published data only}
-
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine‐haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology 1996;15(5):429‐36. - PubMed
Ortega‐Soto 1993 {published data only}
-
- Ortega‐Soto HA, Fernandez AE, Pinedo H, Torre P, Brunner E, Apiquian R. Clinical trial of haloperidol threshold doses. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; 1993 May 22‐27; San Francisco. 1993.
Ortega‐Soto 1994 {published data only}
-
- Ortega‐Soto HA, Brunner E, Apiquian R, Torre P. Therapeutic minimum dose of haloperidol in schizophrenia. Neuropsychopharmocology 1994;10(3S Pt 2):140S.
-
- Ortega‐Soto HA, Fernandez AE, Pinedo H, Torre P, Brunner E, Apiquian R. Clinical trial of haloperidol threshold doses. Patient Care for the 21st Century: Asserting Professional Values with Economic Restraints. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; 1993, May 22‐27; San Francisco. 1993.
Reschke 1974 {published data only}
-
- Reschke RW. Parenteral haloperidol for rapid control of severe, disruptive symptoms of acute schizophrenia. Diseases of the Nervous System 1974;35(3):112‐5. [MEDLINE: ] - PubMed
Santos 1989 {published data only}
-
- Santos JL, Cabranes JA, Almoguera I, Ramos JA, Vazquez C, Angeles F. Clinical implications of determination of plasma haloperidol levels. Acta Psychiatrica Scandinavica 1989;79(4):348‐54. - PubMed
-
- Santos JL, Cabranes JA, Vazquez C, Fuentenebro F, Almoguera I, Ramos JA. Clinical response and plasma haloperidol levels in chronic and subchronic schizophrenia. Biological Psychiatry 1989;26(4):381‐8. - PubMed
-
- Santos JL, Ramos JA, Prieto P, Almoguera I, Vazquez C, Rubio ME, et al. Determination of plasma haloperidol concentrations by radioreceptor assay in schizophrenia‐clinical utility. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 1989;13(6):917‐25. - PubMed
Sim 1989 {published data only}
-
- Sim CB, Lee YC, Hwang JP. Haloperidol for schizophrenic inpatients with three doseage regimens. Psychiatry Today: Accomplishments and Promises; Proceedings of the 8th World Congress of Psychiatry; 1989, Oct 12‐19; Athens, Greece. London: Excerpta Medica International Congress Series 899, 1989:817.
Smith 1984 {published data only}
-
- Smith RC. Plasma haloperidol levels‐clinical‐response and fancy mathematics ‐ reply. Archives of General Psychiatry 1985;42(8):835‐8. - PubMed
-
- Smith RC. The interpretation of plasma haloperidol concentrations‐reply. Archives of General Psychiatry 1985;42(8):839‐40. - PubMed
-
- Smith RC, Baumgartner R, Burd A, Calderon M, Largen J, Mauldin M, et al. Cortical atrophy and white matter density in the brains of schizophrenics and clinical‐response to neuroleptics. Acta Psychiatrica Scandinavica 1987;75(1):11‐9. - PubMed
-
- Smith RC, Baumgartner R, Misra CH, Mauldin M, Shvartsburd A, Ho BT, et al. Haloperidol. Plasma levels and prolactin response as predictors of clinical improvement in schizophrenia: chemical v radioreceptor plasma level assays. Archives of General Psychiatry 1984;41(11):1044‐9. - PubMed
-
- Smith RC, Burd A, Baumgartner R, Ravichandran GK, Mauldin M. Haloperidol and thioridazine drug levels and clinical‐response in schizophrenia‐comparison of gas‐liquid‐chromatography and radioreceptor drug level assays. Psychopharmacology Bulletin 1985;21(1):52‐8. - PubMed
Smith 1987 {published data only}
-
- Smith RC. Plasma haloperidol levels and clinical response. Archives of General Psychiatry 1987;44(12):1110‐2. - PubMed
Van Putten 1990 {published data only}
-
- Putten T, Marder SR, Mintz J. A controlled dose comparison of haloperidol in newly admitted schizophrenic patients. Archives of General Psychiatry 1990;47(8):754‐8. - PubMed
Volavka 2000 {published data only}
-
- Volavka J, Cooper TB, Czobor P, Lindenmayer J P, Citrome LL, Mohr P, et al. High dose treatment with haloperidol: the effect of dose reduction. Journal of Clinical Psychopharmacology 2000;20(2):252‐6. [MEDLINE: ] - PubMed
-
- Volavka J, Cooper TB, Czobor P, Lindenmayer JP, Citrome LL, Mohr P. Haloperidol blood levels and effects in schizophrenia. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego. 1997.
Zimbroff 1997 {published data only}
-
- Baker R, Mack R, Morris D, Sebree T, Kashkin KB. The efficacy and safety of three doses of sertindole versus three doses of haloperidol in schizophrenic patients. Proceedings of the 9th European College of Neuropsychopharmacology Congress; 1996 Sep 21‐25; Amsterdam, Netherlands. 1996.
-
- Bark N, Mack R, Zborowski J, Morris D, Sebree T, Shook S, et al. Efficacy and safety of three doses of sertindole and haloperidol in schizophrenic patients. Biological Psychiatry 1996;39:597.
-
- Larson GL, Mack RJ, Zborowski J, Morris DD, Sebree TB, Wallin BA. Three doses each of sertindole and haloperidol in schizophrenics. Proceedings of the 10th World Congress of Psychiatry; 1996 Aug 23‐28; Madrid, Spain. 1996.
-
- Tamminga C, Mack R, Zborowski J, Morris D, Sebree T, Wallin B. Efficacy and safety of three doses of sertindole and haldol in schizophrenic patients. Proceedings of the 20th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1996 Jun 23‐27; Melbourne, Australia. 1996.
-
- Zimbroff DL, Kane JM, Tamminga C, Daniel DG, Mack RJ, Wozniak PJ, et al. Controlled, dose‐response study of sertindole and haloperidol in the treatment of schizophrenia. Sertindole study group. American Journal of Psychiatry 1997;154(6):782‐91. - PubMed
Additional references
Addington 1990
-
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophrenia Research 1990;3:247‐51. - PubMed
Altman 1996
APA 1997
-
- American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. American Journal of Psychiatry 1997;154 Suppl 4:1‐63. - PubMed
Awad 1993
-
- Awad AG. Methodological and design issues in clinical trials of new neuroleptics: an overview. British Journal of Psychiatry 1993;Suppl 22:51‐7. - PubMed
Awad 1999
-
- Awad AG, Voruganti LP. Cost‐utility analysis in schizophrenia. Journal of Clinical Psychiatry 1999;60 Suppl 3:22‐6. - PubMed
Bagnall 2002
Baldessarini 1984
-
- Baldessarini RJ, Katz B, Cotton P. Dissimilar dosing with high‐potency and low‐potency neuroleptics. American Journal of Psychiatry 1984;141(6):748‐52. - PubMed
Baldessarini 1988
-
- Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacologic treatment of psychoses. Archives of General Psychiatry 1988;45:79‐91. - PubMed
Baldessarini 1995
-
- Baldessarini RJ, Kando JC, Centorrino F. Hospital use of antipsychotic agents in 1989 and 1993: stable dosing with decreased length of stay. American Journal of Psychiatry 1995;152(7):1038‐44. - PubMed
Bezchlibnyk 1994
-
- Bezchlibnyk‐Butler KZ, Remington GJ. Antiparkinsonian drugs in the treatment of neuroleptic‐induced extrapyramidal symptoms. Canadian Journal of Psychiatry 1994;39(2):74‐84. - PubMed
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Bollini 1994
-
- Bollini P, Pampallona S, Orza MJ, Adamas ME, Chalmers TC. Antipsychotic drugs: is more worse? A meta‐analysis of the published randomised control trials. Psychological Medicine 1994;24:307‐16. - PubMed
Citrome 2011
-
- Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved antipsychotic. International Journal of Clinical Practise 2011;65(2):189‐210. - PubMed
Clarke 2002
-
- Clarke M, Oxman AD (editors). Cochrane Reviewer's Handbook. Cochrane Database of Systematic Reviews 2002, issue 2.
Conley 1997
-
- Conley RR, Carpenter WT Jr, Tamminga CA. Time to clozapine response in a standardized trial. American Journal of Psychiatry 1997;154(9):1243‐7. - PubMed
CPA 1998
-
- Canadian Psychiatric Association. Canadian clinical practice guidelines for the treatment of schizophrenia. Canadian Journal of Psychiatry‐Revue Canadienne De Psychiatrie 1998;2 Suppl 43:25‐40. - PubMed
Davis 2003
-
- Davis JM, Chen N, Glick ID. A meta‐analysis of the efficacy of second‐generation antipsychotics. Archives General Psychiatry 2003;60(6):553‐64. - PubMed
De Oliveira 1996
-
- Oliveira IR, Sena EP, Pereira EL. Haloperidol blood levels and clinical outcome: a meta‐analysis of studies relevant to testing the therapeutic window hypothesis. Journal of Clinical Pharmacy and Therapeutics 1996;21(4):229‐36. - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Abstracts of 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town, South Africa. 2000.
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomised trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Duggan 2002
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: sulpiride versus placebo for schizophrenia 18/22 methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Emsley 1995
-
- Emsley R, McCreadie R, Livingston M, Smedt G, Lemmens P. Risperidone in the treatment of first‐episode schizophrenic patients with schizophreniform disorder: a double blind study. Congress of the International Academy for Biomedical and Drug Research on Critical Issues in the Treatment of Schizophrenia. 1995.
Endicott 1978
-
- Endicott J, Spitzer RL. A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Archives of General Psychiatry 1978;35:837‐44. - PubMed
Gardner 2005
Geddes 1999
-
- Geddes J. Royal College of Psychiatrists guidelines on for the care of those with schizophrenia. The Schizophrenia Trials Meeting; 1999 May 5‐7; Stratford‐upon‐Avon, UK 1999.
Glazer 1998
-
- Glazer WM. Formulary decisions and health economics. Journal of Clinical Psychiatry 1998;59 Suppl 19:23‐9. - PubMed
Gulliford 1999
-
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised. Rockville: National Institute of Mental Health, 1976.
Hansen 1997
-
- Hansen TE, Casey DE, Hoffman WF. Neuroleptic intolerance. Schizophrenia Bulletin 1997;23(4):567‐82. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hogarty 1973
-
- Hogarty GE, Goldberg SC. Drug and sociotherapy in the aftercare of schizophrenic patients. One‐year relapse rates. Archives of General Psychiatry 1973;28(1):54‐64. - PubMed
Hogarty 1974
-
- Hogarty GE, Goldberg SC, Schooler NR, Ulrich RF. Drug and sociotherapy in the aftercare of schizophrenic patients. II. Two‐year relapse rates. Archives of General Psychiatry 1974;31(5):603‐8. - PubMed
Hoyberg 1993
-
- Hoyberg O, Fensbo C, Remvig J, LingJaerde O, Sloth‐Neilsen M, Salvesen I. Risperidone versus perphenazine in the treatment of chronic schizophrenic patients with acute exacerbations. Acta Psychiatrica Scandinavica 1993;88:395‐402. - PubMed
Hutton 2009
-
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. - PubMed
Huttunen 1995
-
- Huttunen M, Piepponen T, Rantanen H, Larmo I, Nyholm R, Raitasuo V. Risperidone versus zuclopenthixol in the treatment of acute schizophrenic episodes: a double‐blind parallel group trial. Acta Psychiatrica Scandinavica 1995;91:271‐7. - PubMed
Jadad 1996
-
- Jadad A, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavanagh DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Jadad 1999
-
- Jadad A. Meta‐analysis making the same mistakes as trials. Proceedings of the 2nd Symposium on Systematic Reviews: Beyond the Basics; 1999 Jan 5‐7; Oxford, UK. 1999.
Johnson 1993
-
- Johnson RE, McFarland BH. Antipsychotic drug exposure in a health maintenance organization. Medical Care 1993;31(5):432‐44. - PubMed
Joy 2002
-
- Joy C, Lawrie S. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003082.pub2] - DOI
Kane 1996
-
- Kane JM. Schizophrenia. New England Journal of Medicine 1996;334(1):34‐41. - PubMed
Kapur 1996
-
- Kapur S, Remington G, Jones C, Wilson A, DaSilva J, Houle S, et al. High levels of dopamine D2 receptor occupancy with low‐dose haloperidol treatment: a PET study. American Journal of Psychiatry 1996;153(7):948‐50. - PubMed
Kapur 1997
-
- Kapur S, Zipursky R, Remington G, Jones C, McKay G, Houle S. PET evidence that loxapine is an equipotent blocker of 5‐HT2 and D2 receptors: implications for the therapeutics of schizophrenia. American Journal of Psychiatry 1997;154(11):1525‐9. - PubMed
Kapur 1998
-
- Kapur S. A new framework for investigating antipsychotic action in humans: lessons from pet imaging. Molecular Psychiatry 1998;3(2):135‐40. - PubMed
Kapur 2001
-
- Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. American Journal of Psychiatry 2001;158(3):360‐9. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986.
Kennedy 2002
Kiivet 1995
Kudo 1999
-
- Kudo Y, Nomura J, Ikawa G, Nakajima T, Saito M, Sakai T, et al. Clinical trial of quetiapine in schizophrenia‐efficacy and tolerability of quetiapine: a comparative double‐blind study with mosapramine in schizophrenic patients. Proceeding of the Annual Meeting of the World Psychiatric Association; 1999 Aug; Hamburg, Germany. 1999.
Leucht 1999
-
- Leucht S, Pitschel‐Walz G, Abraham D, Kissling W. Efficacy and extra‐pyramidal side‐effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta‐analysis of randomised controlled trials. Schizophrenia Research 1999;35:51‐68. - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. British Journal of Psychiatry 2005;187:366‐71. - PubMed
Leucht 2005b
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. - PubMed
Levy 1996
-
- Levy RH. Sedation in acute and chronic agitation. Pharmacotherapy 1996;6(Part 2):152‐9S, 166‐8S. - PubMed
Lieberman 1993
-
- Lieberman J, Jody D, Geisler S. Time course and biologic correlates of treatment response in first‐episode schizophrenia. Archives of General Psychiatry 1993;50(5):369‐76. - PubMed
Marder 1998
-
- Marder SR. Facilitating compliance with antipsychotic medication. Journal of Clinical Psychiatry 1998;59(Suppl 3):21‐5. - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Miller 1997
-
- Miller R. Dose‐response relationships for the antipsychotic effects and parkinsonian side‐effects of typical neuroleptic drugs: practical and theoretical implications. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 1997;21(7):1059‐94. - PubMed
Milton 1995
-
- Milton GV, Jann MW. Emergency treatment of psychotic symptoms. Pharmacokinetic considerations for antipsychotic drugs. Clinical Pharmacokinetics 1995;28(6):494‐504. - PubMed
Murasaki 1999
-
- Murasaki M, Koyama T, Yamauchi T, Yagi MG, Ushijima S, Kamijima K. Clinical evaluation of quetiapine in schizophrenia‐efficacy and tolerability of quetiapine compared with haloperidol in patients with schizophrenia. Proceedings of the 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg, Germany. 1999.
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812.
Peuskens 1997
-
- Peuskens J, Link CGG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatrica Scandinavica 1997;96:265‐73. - PubMed
Raschetti 1993
-
- Raschetti R, Spila Alegiani S, Diana G, Cas R, Traversa G, Pasquini P. Antipsychotic drug prescription in general practice in Italy. Acta Psychiatrica Scandinavica 1993;87(5):317‐21. - PubMed
Reardon 1989
-
- Reardon GT, Rifkin A, Schwartz A, Myerson A, Siris SG. Changing patterns of neuroleptic dosage over a decade. American Journal of Psychiatry 1989;146(6):726‐9. - PubMed
Rosenheck 2003
-
- Rosenheck MD, Perlick D, Bingham S, Liu‐Mares W, Collins J, Warren S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia. JAMA 2003;290(20):2693‐702. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011:359‐83.
Simpson 1970
-
- Simpson M, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;212:11‐9. - PubMed
Srisurapanont 2002
-
- Srisurapanont M, Disayavanish C, Taimkaew K. Quetiapine for schizophrenia. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD000967.pub2] - DOI
Thornley 2002
Thornley 1998
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
Whicher 2002
WHO 2011
-
- World Health Organization. WHO Model Lists of Essential Medicines, 2011 www.who.int/medicines/publications/essentialmedicines/en/. (accessed 20 June 2013).
Williams 1999
-
- Williams CL, Johnstone BM, Kesterson JG, Javor KA, Schmetzer AD. Evaluation of antipsychotic and concomitant medication use patterns in patients with schizophrenia. Medical Care 1999;37 Suppl 4:81‐6. - PubMed
Zito 1998
-
- Zito JM. Pharmacoeconomics of the new antipsychotics for the treatment of schizophrenia. Psychiatric Clinics of North America 1998;21(1):181‐202. - PubMed
References to other published versions of this review
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

