Purification and Characterization of Intracellular Cellulase from Aspergillus oryzae ITCC-4857.01
- PMID: 23983520
- PMCID: PMC3749401
- DOI: 10.4489/MYCO.2009.37.2.121
Purification and Characterization of Intracellular Cellulase from Aspergillus oryzae ITCC-4857.01
Abstract
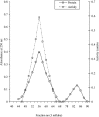
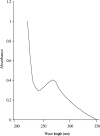
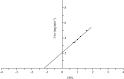
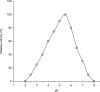
Purification and characterization of intracellular cellulase produced by A. oryzae ITCC-4857.01 are reported. The enzyme was purified by ion-exchange chromatography using DEAE-cellulose followed by Gel filtration. The purification achieved was 41 fold from the crude extract with yield of 27%. The purified enzyme showed single band on poly acrylamide gel. The molecular weight as determined by SDS-PAGE and gel filtration was 38 KDa and 38.6 KDa respectively and contained only one subunit. The enzyme is glycoprotien as nature and contained 0.67% neutral sugar. The apparent Km value of the enzyme against cellulose was 0.83%. The enzyme showed the highest relative ativities on CMC followed by avicel, salicin and filter paper. The optimum pH of activity was 5.5 and very slight activity was observed at or above pH 7.5 as well as bellow pH 3.5. The optimum tempreture of the activity was 45℃ and the highest activity was exhibited in 35 to 45℃. The enzyme lost their activities almost completely (95~100%) at 80 ℃ or above and as well as bellow 25℃.
Keywords: Aspergillus; DEAE-cellulose chromatography; Gel filtration; Intracellular cellulase; SDS-PAGE.
Figures




References
-
- Akiba S, Kimura Y, Yamamoto K, Kumagai H. Purification and characterization of a protease-resistant cellulase from Aspergillus niger. J Ferment Bioeng. 1995;79:125–130.
-
- Amadioha AC. Production of cellulolytic enzymes by Rhizopus oryzae in culture and Rhizopus-infected tissues of potato tubers. Mycologia. 1993;88:574–578.
-
- Beguin P. Molecular biology of cellulose degradation. Annu Rev Microbiol. 1990;44:219–248. - PubMed
-
- Begum MF. Screening of aspergilli from cellulosic waste materials and studies on their cellulolytic properties. Bangladesh: Department of Botany, University of Rajshahi; 2005. Ph.D. thesis.
LinkOut - more resources
Full Text Sources
