Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor
- PMID: 23983930
- PMCID: PMC3736894
- DOI: 10.1177/2045125311409486
Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor
Abstract
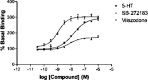
Background: Vilazodone is the latest US Food and Drug Administration approved antidepressant agent available in the USA. Its putative mechanism of antidepressant action enhances the release of serotonin across the brain's serotonergic pathways specifically by inhibiting the serotonin transporter, similar to a selective serotonin reuptake inhibitor (SSRI), and simultaneously stimulating serotonin-1a receptors via partial agonism, similar to the anxiolytic bus-pirone. This combined activity in the single vilazodone agent has been termed by the authors as being a serotonin partial agonist and reuptake inhibitor or (SPARI).
Methods: A MEDLINE and Internet search was conducted and the resultant preclinical and clinical evidence was reviewed. The authors attempt to review laboratory data, animal model data and human trial data to develop a translational theory on the mechanism of antidepressant action of this agent and also its adverse effect potential.
Results: Randomized, controlled empirical data for vilazodone have gained it approval for treating major depressive disorder. It combines two well known pharmacodynamic mechanisms of serotonergic action into a novel agent. Although no head-to-head studies against other antidepressants have been published, the efficacy data for vilazodone appear comparable to other known antidepressants, with similar gastrointestinal side effects to SSRI or serotonin norepinephrine reuptake inhibitor (SNRI) antidepressants, but possibly with a lower incidence of sexual side effects and weight gain.
Discussion: Vilazodone will lend itself to the current armamentarium in the treatment of major depressive disorder and may hold promise for patients who cannot tolerate other antide-pressants. Its unique SPARI mechanism of action could also be efficacious for patients who do not respond to SSRI or SNRI antidepressant monotherapies.
Keywords: antidepressant; major depressive disorder; serotonin; vilazodone.
Conflict of interest statement
Thomas L. Schwartz, MD is an associate professor of psychiatry at the SUNY Upstate Medical University. Over the past 12 months (May 2010-May 2011) Dr Schwartz has served as a Consultant to PamLab. He has served on speakers bureaus for Pfizer Inc., Wyeth Pharmaceuticals, AstraZeneca, and Merck, and has received research and/or grant support from Cephalon, Cyberonics, and Forest.
Umar Siddiqui, MD is a research coordinator at the SUNY Upstate Medical University's Treatment Resistant Depression and Anxiety Disorders Program. He has no conflicts of interest to disclose.
Stephen M. Stahl, MD, PhD is an adjunct professor of psychiatry at the University of California, San Diego School of Medicine and an honorary visiting senior fellow at the University of Cambridge, UK. Over the past 12 months (January 2009-January 2010) Dr Stahl has served as a Consultant to Allergan, Astra Zeneca, BioMarin, BioVail, Boehringer Ingelheim, Bristol Myers-Squibb, Cenerex, Covance, Cypress Bioscience, Dianippon, Eisai, Eli Lilly, Forest, GlaxoSmith Kline, Labopharm, Lundbeck, Marinus, Meda Corp, Meiji, Merck, Novartis, Pfizer, Pfizer Canada, Pierre Fabre, PamLab, Prexa Pharmaceuticals, Propagate Pharma, Royalty Pharma, Sanofi, Schering Plough Corporation, Shire, SK Corporation, Soffinova, Solvay, Vanda, and Wyeth. He has served on speakers bureaus for Pfizer Inc., Wyeth Pharmaceuticals and Schering Plough Corporation and has received research and/or grant support from Astra Zeneca, Boehringer Ingelheim, Bristol Myers-Squibb, Cephalon, Dainippon, Eli Lilly, Forest, Lundbeck, Novartis, PamLabs, Pfizer, Pfizer Canada, Pharmasquire, Sanofi Aventis, Schering Plough, Shire, and Wyeth.
Figures

References
-
- Adamec R., Bartoszyk G., Burton P. (2004) Effects of systemic injections of vilazodone, a selective serotonin reuptake inhibitor and serotonin 1A receptor agonist, on anxiety induced by predator stress in rats. Eur J Pharmacol 504: 65–77 - PubMed
-
- APA. (2000) American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th edn text revision, American Psychiatric Publishing: Washington, DC
-
- APA. (2010) American Psychiatric Association practice guideline for major depressive disorder in adults. Am J Psychiatry 150: 1–26 - PubMed
-
- Bartoszyk G.D., Hegenbart R., Ziegler H. (1997) EMD 68843, a serotonin reuptake inhibitor with selective presynaptic 5-HT1A receptor agonistic properties. Eur J Pharmacol 322: 147–153 - PubMed
LinkOut - more resources
Full Text Sources

