The down-regulation of GNAO1 and its promoting role in hepatocellular carcinoma
- PMID: 23984917
- PMCID: PMC3775511
- DOI: 10.1042/BSR20130001
The down-regulation of GNAO1 and its promoting role in hepatocellular carcinoma
Abstract
GNAO1 (guanine nucleotide-binding protein, α-activating activity polypeptide O) is a member of the subunit family of Gα proteins, which are molecular switchers controlling signal transductions and whose deregulation can promote oncogenesis. HCC (hepatocellular carcinoma) is one of the malignant tumours around the world, which summons novel biomarkers or targets for effective diagnosis and treatments. The present study was aimed to investigate the expression of GNAO1 in HCC patient tissues and the possible mechanisms by which it took effects. The expression of GNAO1 was detected by IHC (immunohistochemistry) and real-time qPCR (quantitative PCR). Cell proliferation test and cell senescence test were then performed to explore the role of GNAO1 in the occurrence and development of HCC. It was revealed that the level of GNAO1 was comparably less in HCC tissues than in the adjacent tissues. Furthermore, down-regulation of GNAO1 increased cell proliferation, while suppressing the senescence of HCC cells. In conclusion, our findings revealed and confirmed the importance of GNAO1 in HCC, indicating that GNAO1 is a potential biomarker as well as a promising therapeutic target for HCC.
Figures

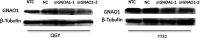


References
-
- Parkin D. M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. - PubMed
-
- Kest B., Smith S. B., Schorscher-Petcu A., Austin J. S., Ritchie J., Klein G., Rossi G. C., Fortin A., Mogil J. S. GNAO1 (G αO protein) is a likely genetic contributor to variation in physical dependence on opioids in mice. Neuroscience. 2009;162:1255–1264. - PubMed
-
- Camateros P., Marino R., Fortin A., Martin J. G., Skamene E., Sladek R., Radzioch D. Identification of novel chromosomal regions associated with airway hyperresponsiveness in recombinant congenic strains of mice. Mamm. Genome. 2010;21:28–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

