Lipid dependencies, biogenesis and cytoplasmic micellar forms of integral membrane sugar transport proteins of the bacterial phosphotransferase system
- PMID: 23985145
- PMCID: PMC3836488
- DOI: 10.1099/mic.0.070953-0
Lipid dependencies, biogenesis and cytoplasmic micellar forms of integral membrane sugar transport proteins of the bacterial phosphotransferase system
Abstract
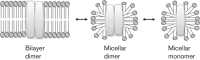
Permeases of the prokaryotic phosphoenolpyruvate-sugar phosphotransferase system (PTS) catalyse sugar transport coupled to sugar phosphorylation. The lipid composition of a membrane determines the activities of these enzyme/transporters as well as the degree of coupling of phosphorylation to transport. We have investigated mechanisms of PTS permease biogenesis and identified cytoplasmic (soluble) forms of these integral membrane proteins. We found that the catalytic activities of the soluble forms differ from those of the membrane-embedded forms. Transport via the latter is much more sensitive to lipid composition than to phosphorylation, and some of these enzymes are much more sensitive to the lipid environment than others. While the membrane-embedded PTS permeases are always dimeric, the cytoplasmic forms are micellar, either monomeric or dimeric. Scattered published evidence suggests that other integral membrane proteins also exist in cytoplasmic micellar forms. The possible functions of cytoplasmic PTS permeases in biogenesis, intracellular sugar phosphorylation and permease storage are discussed.
Figures

Similar articles
-
Subcellular localization and logistics of integral membrane protein biogenesis in Escherichia coli.J Mol Microbiol Biotechnol. 2013;23(1-2):24-34. doi: 10.1159/000346517. Epub 2013 Apr 18. J Mol Microbiol Biotechnol. 2013. PMID: 23615193 Review.
-
Soluble sugar permeases of the phosphotransferase system in Escherichia coli: evidence for two physically distinct forms of the proteins in vivo.Mol Microbiol. 2003 Apr;48(1):131-41. doi: 10.1046/j.1365-2958.2003.03394.x. Mol Microbiol. 2003. PMID: 12657050
-
Protein:Protein interactions in the cytoplasmic membrane apparently influencing sugar transport and phosphorylation activities of the e. coli phosphotransferase system.PLoS One. 2019 Nov 21;14(11):e0219332. doi: 10.1371/journal.pone.0219332. eCollection 2019. PLoS One. 2019. PMID: 31751341 Free PMC article.
-
Dependency of sugar transport and phosphorylation by the phosphoenolpyruvate-dependent phosphotransferase system on membranous phosphatidylethanolamine in Escherichia coli: studies with a pssA mutant lacking phosphatidylserine synthase.Arch Microbiol. 2004 Jan;181(1):26-34. doi: 10.1007/s00203-003-0623-7. Epub 2003 Nov 21. Arch Microbiol. 2004. PMID: 14634719
-
Carbohydrate Transport by Group Translocation: The Bacterial Phosphoenolpyruvate: Sugar Phosphotransferase System.Subcell Biochem. 2019;92:223-274. doi: 10.1007/978-3-030-18768-2_8. Subcell Biochem. 2019. PMID: 31214989 Review.
Cited by
-
A Novel Iron Transporter SPD_1590 in Streptococcus pneumoniae Contributing to Bacterial Virulence Properties.Front Microbiol. 2018 Jul 20;9:1624. doi: 10.3389/fmicb.2018.01624. eCollection 2018. Front Microbiol. 2018. PMID: 30079056 Free PMC article.
-
An acid-tolerance response system protecting exponentially growing Escherichia coli.Nat Commun. 2020 Mar 20;11(1):1496. doi: 10.1038/s41467-020-15350-5. Nat Commun. 2020. PMID: 32198415 Free PMC article.
-
EⅡB Mutation Reduces the Pathogenicity of Listeria monocytogenes by Negatively Regulating Biofilm Formation Ability, Infective Capacity, and Virulence Gene Expression.Vet Sci. 2024 Jul 2;11(7):301. doi: 10.3390/vetsci11070301. Vet Sci. 2024. PMID: 39057985 Free PMC article.
-
Integrated Translatomics with Proteomics to Identify Novel Iron-Transporting Proteins in Streptococcus pneumoniae.Front Microbiol. 2016 Feb 3;7:78. doi: 10.3389/fmicb.2016.00078. eCollection 2016. Front Microbiol. 2016. PMID: 26870030 Free PMC article.
References
-
- Aboulwafa M., Saier M. H., Jr (2002). Dependency of sugar transport and phosphorylation by the phosphoenolpyruvate-dependent phosphotransferase system on membranous phosphatidyl glycerol in Escherichia coli: studies with a pgsA mutant lacking phosphatidyl glycerophosphate synthase. Res Microbiol 153, 667–677. 10.1016/S0923-2508(02)01376-1 - DOI - PubMed
-
- Aboulwafa M., Saier M. H., Jr (2007). In vitro interconversion of the soluble and membrane-integrated forms of the Escherichia coli glucose enzyme II of the phosphoenolpyruvate-dependent sugar-transporting phosphotransferase system. J Mol Microbiol Biotechnol 12, 263–268. 10.1159/000099647 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

