National and state vaccination coverage among adolescents aged 13-17 years--United States, 2012
- PMID: 23985496
- PMCID: PMC4604993
National and state vaccination coverage among adolescents aged 13-17 years--United States, 2012
Abstract
At ages 11 through 12 years, the Advisory Committee on Immunization Practices (ACIP) recommends that preteens receive 1 dose of tetanus, diphtheria, and acellular pertussis (Tdap) vaccine, 1 dose of meningococcal conjugate (MenACWY) vaccine, and 3 doses of human papillomavirus (HPV) vaccine. ACIP recommends administration of all age-appropriate vaccines during a single visit. ACIP also recommends that pre-teens and older adolescents receive an annual influenza vaccine as well as any overdue vaccines (e.g., varicella). To monitor vaccination coverage among persons aged 13-17 years, CDC analyzed data from the National Immunization Survey-Teen (NIS-Teen). This report highlights findings of that analysis. From 2011 to 2012, coverage increased for ≥1 Tdap vaccine dose (from 78.2% to 84.6%), ≥1 MenACWY vaccine dose (from 70.5% to 74.0%) and, among males, ≥1 HPV vaccine dose (from 8.3% to 20.8%). Among females, vaccination coverage estimates for each HPV vaccine series dose were similar in 2012 compared with 2011. Coverage varied substantially among states. Regarding Healthy People 2020 targets for adolescents, 36 states achieved targets for Tdap, 12 for MenACWY, and nine for varicella vaccine coverage. Large and increasing coverage differences between Tdap and other vaccines recommended for adolescents indicate that substantial missed opportunities remain for vaccinating teens, especially against HPV infection. Health-care providers should administer recommended HPV and meningococcal vaccinations to boys and girls during the same visits when Tdap vaccine is given. In addition, whether for health problems or well-checks, providers, parents, and adolescents should use every health-care visit as an opportunity to review adolescents' immunization histories and ensure that every adolescent is fully vaccinated.
Figures
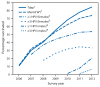
References
-
- CDC. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years and adults aged 19 years and older—United States, 2013. MMWR. 2013;62(Suppl 1):1–19. - PubMed
-
- CDC. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2007;56(RR-2):1–24. - PubMed
-
- CDC. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. 2011;60:1705–8. - PubMed
-
- CDC. General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices. MMWR. 2011;60(RR-2) - PubMed
-
- US Department of Health and Human Services. Healthy people 2020. Washington, DC: US Department of Health and Human Services; 2012. Available at http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.as....

