Mosquito larval source management for controlling malaria
- PMID: 23986463
- PMCID: PMC4669681
- DOI: 10.1002/14651858.CD008923.pub2
Mosquito larval source management for controlling malaria
Update in
-
Mosquito aquatic habitat modification and manipulation interventions to control malaria.Cochrane Database Syst Rev. 2022 Nov 11;11(11):CD008923. doi: 10.1002/14651858.CD008923.pub3. Cochrane Database Syst Rev. 2022. PMID: 36367444 Free PMC article.
Abstract
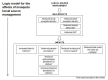
Background: Malaria is an important cause of illness and death in people living in many parts of the world, especially sub-Saharan Africa. Long-lasting insecticide treated bed nets (LLINs) and indoor residual spraying (IRS) reduce malaria transmission by targeting the adult mosquito vector and are key components of malaria control programmes. However, mosquito numbers may also be reduced by larval source management (LSM), which targets mosquito larvae as they mature in aquatic habitats. This is conducted by permanently or temporarily reducing the availability of larval habitats (habitat modification and habitat manipulation), or by adding substances to standing water that either kill or inhibit the development of larvae (larviciding).
Objectives: To evaluate the effectiveness of mosquito LSM for preventing malaria.
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; EMBASE; CABS Abstracts; and LILACS up to 24 October 2012. We handsearched the Tropical Diseases Bulletin from 1900 to 2010, the archives of the World Health Organization (up to 11 February 2011), and the literature database of the Armed Forces Pest Management Board (up to 2 March 2011). We also contacted colleagues in the field for relevant articles.
Selection criteria: We included cluster randomized controlled trials (cluster-RCTs), controlled before-and-after trials with at least one year of baseline data, and randomized cross-over trials that compared LSM with no LSM for malaria control. We excluded trials that evaluated biological control of anopheline mosquitoes with larvivorous fish.
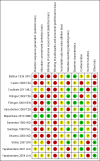
Data collection and analysis: At least two authors assessed each trial for eligibility. We extracted data and at least two authors independently determined the risk of bias in the included studies. We resolved all disagreements through discussion with a third author. We analyzed the data using Review Manager 5 software.
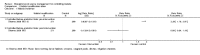
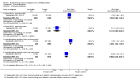
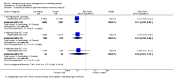
Main results: We included 13 studies; four cluster-RCTs, eight controlled before-and-after trials, and one randomized cross-over trial. The included studies evaluated habitat modification (one study), habitat modification with larviciding (two studies), habitat manipulation (one study), habitat manipulation plus larviciding (two studies), or larviciding alone (seven studies) in a wide variety of habitats and countries. Malaria incidenceIn two cluster-RCTs undertaken in Sri Lanka, larviciding of abandoned mines, streams, irrigation ditches, and rice paddies reduced malaria incidence by around three-quarters compared to the control (RR 0.26, 95% CI 0.22 to 0.31, 20,124 participants, two trials, moderate quality evidence). In three controlled before-and-after trials in urban and rural India and rural Kenya, results were inconsistent (98,233 participants, three trials, very low quality evidence). In one trial in urban India, the removal of domestic water containers together with weekly larviciding of canals and stagnant pools reduced malaria incidence by three quarters. In one trial in rural India and one trial in rural Kenya, malaria incidence was higher at baseline in intervention areas than in controls. However dam construction in India, and larviciding of streams and swamps in Kenya, reduced malaria incidence to levels similar to the control areas. In one additional randomized cross-over trial in the flood plains of the Gambia River, where larval habitats were extensive and ill-defined, larviciding by ground teams did not result in a statistically significant reduction in malaria incidence (2039 participants, one trial). Parasite prevalenceIn one cluster-RCT from Sri Lanka, larviciding reduced parasite prevalence by almost 90% (RR 0.11, 95% CI 0.05 to 0.22, 2963 participants, one trial, moderate quality evidence). In five controlled before-and-after trials in Greece, India, the Philippines, and Tanzania, LSM resulted in an average reduction in parasite prevalence of around two-thirds (RR 0.32, 95% CI 0.19 to 0.55, 8041 participants, five trials, moderate quality evidence). The interventions in these five trials included dam construction to reduce larval habitats, flushing of streams, removal of domestic water containers, and larviciding. In the randomized cross-over trial in the flood plains of the Gambia River, larviciding by ground teams did not significantly reduce parasite prevalence (2039 participants, one trial).
Authors' conclusions: In Africa and Asia, LSM is another policy option, alongside LLINs and IRS, for reducing malaria morbidity in both urban and rural areas where a sufficient proportion of larval habitats can be targeted. Further research is needed to evaluate whether LSM is appropriate or feasible in parts of rural Africa where larval habitats are more extensive.
Conflict of interest statement
Ulrike Fillinger, John Gimnig and Steve Lindsay have been the primary investigators and authors of studies that were reviewed. They did not review their own studies. Ulrike Fillinger, Steve Lindsay and Lucy Tusting have received financial support from Valent BioSciences Corporation, USA, a manufacturer of microbial larvicides. Valent BioSciences Corporation had no involvement in the data analysis or preparation of the final report. We have no other interests to disclose.
Figures















References
References to studies included in this review
-
- Balfour MC. Some features of malaria in Greece and experience with its control. Rivista di Malariologia 1936;15:114‐131.
-
- Coulibaly MB, Pharm D. Integrated vector management: Impact of the combination of larval control and Indoor Residual Spraying on Anopheles gambiae density and vector capacity for human malaria. Unpublished data2011.
References to studies excluded from this review
-
- Anonymous. Papers on mosquito control. Mosquito News 1944;4(3):65‐101.
-
- Anonymous. First conference of the U.S.S.R. for aeroplane use in malaria control. Meditsinskaya Parazitologiya i Parazitarnye Bolezni. 1932; Vol. 1:2.
-
- Anonymous. Report of the Bureau of Malaria Control 1926‐27. Rep Comm Hlth Porto Rico 1929:62‐95.
-
- Anonymous. A review of the control of malaria in Palestine (1918‐1941). A Review of the Control of Malaria in Palestine 1941;40.
-
- Buduilin VG, Andreeva VV. Paris Green as a larvicide in the control of malaria. Tropicheskaya Meditsina i Veterinariya 1931;9(9):464‐9.
Additional references
-
- Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. American Journal of Tropical Medicine and Hygiene 1999;61(1):109‐13. - PubMed
-
- Bockarie MJ, Gbakima AA, Barnish G. It all began with Ronald Ross: 100 years of malaria research and control in Sierra Leone (1899‐1999). Annals of Tropical Medicine and Parasitology 1999;93(3):213‐4. - PubMed
-
- Bogh C, Clarke S, Jawara M, Thomas CJ, Lindsay SW. Localized breeding of the Anopheles gambiae complex (Diptera: Culicidae) along the River Gambia, West Africa. Bulletin of Entomological Research 2003;93(4):279‐87. - PubMed
-
- Bøgh C, Lindsay SW, Clarke SE, Dean A, Jawara M, Pinder M, et al. High spatial resolution mapping of malaria transmission risk in the Gambia, west Africa, using LANDSAT TM satellite imagery. American Journal of Tropical Medicine and Hygiene 2007;76(5):875‐81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

