Impact of functional MRI data preprocessing pipeline on default-mode network detectability in patients with disorders of consciousness
- PMID: 23986694
- PMCID: PMC3749435
- DOI: 10.3389/fninf.2013.00016
Impact of functional MRI data preprocessing pipeline on default-mode network detectability in patients with disorders of consciousness
Erratum in
- Front Neuroinform. 2014;8:50
Abstract
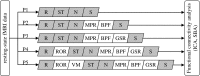
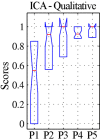
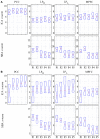
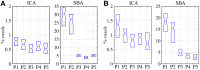
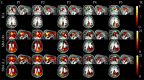
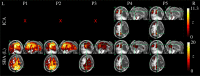
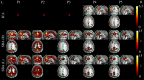
An emerging application of resting-state functional MRI (rs-fMRI) is the study of patients with disorders of consciousness (DoC), where integrity of default-mode network (DMN) activity is associated to the clinical level of preservation of consciousness. Due to the inherent inability to follow verbal instructions, arousal induced by scanning noise and postural pain, these patients tend to exhibit substantial levels of movement. This results in spurious, non-neural fluctuations of the rs-fMRI signal, which impair the evaluation of residual functional connectivity. Here, the effect of data preprocessing choices on the detectability of the DMN was systematically evaluated in a representative cohort of 30 clinically and etiologically heterogeneous DoC patients and 33 healthy controls. Starting from a standard preprocessing pipeline, additional steps were gradually inserted, namely band-pass filtering (BPF), removal of co-variance with the movement vectors, removal of co-variance with the global brain parenchyma signal, rejection of realignment outlier volumes and ventricle masking. Both independent-component analysis (ICA) and seed-based analysis (SBA) were performed, and DMN detectability was assessed quantitatively as well as visually. The results of the present study strongly show that the detection of DMN activity in the sub-optimal fMRI series acquired on DoC patients is contingent on the use of adequate filtering steps. ICA and SBA are differently affected but give convergent findings for high-grade preprocessing. We propose that future studies in this area should adopt the described preprocessing procedures as a minimum standard to reduce the probability of wrongly inferring that DMN activity is absent.
Keywords: data preprocessing; disorders of consciousness; functional MRI (fMRI); functional connectivity; minimally-conscious state; resting-state; vegetative state.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

