Abnormal task modulation of oscillatory neural activity in schizophrenia
- PMID: 23986729
- PMCID: PMC3753429
- DOI: 10.3389/fpsyg.2013.00540
Abnormal task modulation of oscillatory neural activity in schizophrenia
Abstract
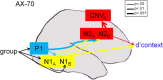
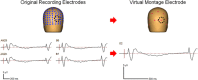
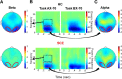
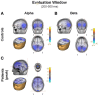
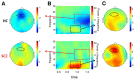
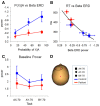
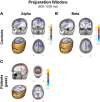
Schizophrenia patients have deficits in cognitive function that are a core feature of the disorder. AX-CPT is commonly used to study cognition in schizophrenia, and patients have characteristic pattern of behavioral and ERP response. In AX-CPT subjects respond when a flashed cue "A" is followed by a target "X," ignoring other letter combinations. Patients show reduced hit rate to "go" trials, and increased false alarms to sequences that require inhibition of a prepotent response. EEG recordings show reduced sensory (P1/N1), as well as later cognitive components (N2, P3, CNV). Behavioral deficits correlate most strongly with sensory dysfunction. Oscillatory analyses provide critical information regarding sensory/cognitive processing over and above standard ERP analyses. Recent analyses of induced oscillatory activity in single trials during AX-CPT in healthy volunteers showed characteristic response patterns in theta, alpha, and beta frequencies tied to specific sensory and cognitive processes. Alpha and beta modulated during the trials and beta modulation over the frontal cortex correlated with reaction time. In this study, EEG data was obtained from 18 schizophrenia patients and 13 controls during AX-CPT performance, and single trial decomposition of the signal yielded power in the target wavelengths. Significant task-related event-related desynchronization (ERD) was observed in both alpha and beta frequency bands over parieto-occipital cortex related to sensory encoding of the cue. This modulation was reduced in patients for beta, but not for alpha. In addition, significant beta ERD was observed over motor cortex, related to motor preparation for the response, and was also reduced in patients. These findings demonstrate impaired dynamic modulation of beta frequency rhythms in schizophrenia, and suggest that failures of oscillatory activity may underlie impaired sensory information processing in schizophrenia that in turn contributes to cognitive deficits.
Keywords: AX-CPT; alpha; beta; cognitive; motor preparation; oscillations; schizophrenia.
Figures


References
-
- Ammons R., Ammons C. (1962). The Quick Test (QT): provisional manual. Psychol. Rep. 11, 111–162
-
- Andreasen N. C. (1984). The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: The University of Iowa - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

