Regulations of gene expression in medullary thymic epithelial cells required for preventing the onset of autoimmune diseases
- PMID: 23986760
- PMCID: PMC3752772
- DOI: 10.3389/fimmu.2013.00249
Regulations of gene expression in medullary thymic epithelial cells required for preventing the onset of autoimmune diseases
Abstract
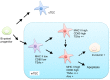
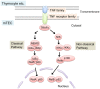
Elimination of potential self-reactive T cells in the thymus is crucial for preventing the onset of autoimmune diseases. Epithelial cell subsets localized in thymic medulla [medullary thymic epithelial cells (mTECs)] contribute to this process by supplying a wide range of self-antigens that are otherwise expressed in a tissue-specific manner (TSAs). Expression of some TSAs in mTECs is controlled by the autoimmune regulator (AIRE) protein, of which dysfunctional mutations are the causative factor of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED). In addition to the elimination of self-reactive T cells, recent studies indicated roles of mTECs in the development of Foxp3-positive regulatory T cells, which suppress autoimmunity and excess immune reactions in peripheral tissues. The TNF family cytokines, RANK ligand, CD40 ligand, and lymphotoxin were found to promote the differentiation of AIRE- and TSA-expressing mTECs. Furthermore, activation of NF-κB is essential for mTEC differentiation. In this mini-review, we focus on molecular mechanisms that regulate induction of AIRE and TSA expression and discuss possible contributions of these mechanisms to prevent the onset of autoimmune diseases.
Keywords: NF-κB; TNF receptor family; autoimmune disease; gene expression; medullary thymic epithelial cells.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

