Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration
- PMID: 23987110
- PMCID: PMC3875778
- DOI: 10.2217/nnm.13.132
Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration
Abstract
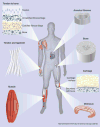
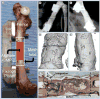
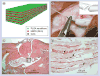
This article reviews recent significant advances in the design of nanofiber scaffolds for orthopedic tissue repair and regeneration. It begins with a brief introduction on the limitations of current approaches for orthopedic tissue repair and regeneration. It then illustrates that rationally designed scaffolds made up of electrospun nanofibers could be a promising solution to overcome the problems that current approaches encounter. The article also discusses the intriguing properties of electrospun nanofibers, including control of composition, structures, orders, alignments and mechanical properties, use as carriers for topical drug and/or gene sustained delivery, and serving as substrates for the regulation of cell behaviors, which could benefit musculoskeletal tissue repair and regeneration. It further highlights a few of the many recent applications of electrospun nanofiber scaffolds in repairing and regenerating various orthopedic tissues. Finally, the article concludes with perspectives on the challenges and future directions for better design, fabrication and utilization of nanofiber scaffolds for orthopedic tissue engineering.
Figures






Similar articles
-
Recent advances in electrospun nanofibers for wound healing.Nanomedicine (Lond). 2017 Jun;12(11):1335-1352. doi: 10.2217/nnm-2017-0017. Epub 2017 May 18. Nanomedicine (Lond). 2017. PMID: 28520509 Free PMC article. Review.
-
In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives.J Biomed Mater Res A. 2022 Feb;110(2):443-461. doi: 10.1002/jbm.a.37290. Epub 2021 Aug 13. J Biomed Mater Res A. 2022. PMID: 34390324 Review.
-
Electrospun nanofibers for regenerative medicine.Adv Healthc Mater. 2012 Jan 11;1(1):10-25. doi: 10.1002/adhm.201100021. Epub 2011 Dec 16. Adv Healthc Mater. 2012. PMID: 23184683 Free PMC article. Review.
-
Small molecule delivery through nanofibrous scaffolds for musculoskeletal regenerative engineering.Nanomedicine. 2014 Nov;10(8):1691-9. doi: 10.1016/j.nano.2014.05.013. Epub 2014 Jun 5. Nanomedicine. 2014. PMID: 24907464 Free PMC article. Review.
-
Immobilization and Application of Electrospun Nanofiber Scaffold-based Growth Factor in Bone Tissue Engineering.Curr Pharm Des. 2015;21(15):1967-78. doi: 10.2174/1381612821666150302152704. Curr Pharm Des. 2015. PMID: 25732663 Review.
Cited by
-
Producing 3D Biomimetic Nanomaterials for Musculoskeletal System Regeneration.Front Bioeng Biotechnol. 2018 Sep 20;6:128. doi: 10.3389/fbioe.2018.00128. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30294596 Free PMC article. Review.
-
A new prototype melt-electrospinning device for the production of biobased thermoplastic sub-microfibers and nanofibers.Biomater Res. 2019 Mar 29;23:10. doi: 10.1186/s40824-019-0159-9. eCollection 2019. Biomater Res. 2019. PMID: 30976458 Free PMC article.
-
Regenerative engineering: a review of recent advances and future directions.Regen Med. 2021 May;16(5):495-512. doi: 10.2217/rme-2021-0016. Epub 2021 May 25. Regen Med. 2021. PMID: 34030463 Free PMC article. Review.
-
Novel PVA/MOF Nanofibres: Fabrication, Evaluation and Adsorption of Lead Ions from Aqueous Solution.Nanoscale Res Lett. 2016 Dec;11(1):414. doi: 10.1186/s11671-016-1631-2. Epub 2016 Sep 20. Nanoscale Res Lett. 2016. PMID: 27644240 Free PMC article.
-
Effect of a New Annular Incision on Biomechanical Properties of the Intervertebral Disc.Orthop Surg. 2016 Feb;8(1):68-74. doi: 10.1111/os.12226. Orthop Surg. 2016. PMID: 27028383 Free PMC article.
References
-
- United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States. 2nd. Vol. 129. American Academy of Orthopaedic Surgeons; IL, USA: 2011.
-
- Deng M, James R, Laurencin CT, Kumbar SG. Nanostructured polymeric scaffolds for orthopaedic regenerative engineering. IEEE Trans Nanobiosci. 2012;11(1):3–14. - PubMed
-
- Talmo CT, Aghazadeh M, Bono JV. Perioperative complications following total joint replacement. Clin Geriatr Med. 2012;28(3):471–487. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
