Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression
- PMID: 23987185
- PMCID: PMC3787467
- DOI: 10.1089/hum.2011.009
Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression
Abstract
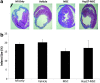
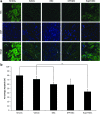
Mesenchymal stem cell (MSC) therapy offers the potential to promote recovery after myocardial infarction (MI). However, therapeutic efficacy may be limited by poor survival and retention of transplanted cells. A combination of gene and cell therapy has the capacity to prevent donor cell death and augment the reparative and regenerative effects of cell transfer. The present study investigates the effect of exogenous heat shock protein 27 (Hsp27) expression in MSCs in an in vitro model of ischemia and in an in vivo rat MI model and aims to determine if this could enhance the therapeutic benefit associated with cell delivery. Hsp27 overexpression by lentivirus vector modification resulted in increased MSC survival in vitro and in vivo. Furthermore, decreased apoptosis in the infarcted tissue and improved cardiac function was observed in the Hsp27 group, enhancing the therapeutic effect of MSCs. Together, these data demonstrate that ex vivo genetic modification-specifically Hsp27 overexpression-offers the possibility of enhancing the efficacy of MSC therapy in MI.
Figures
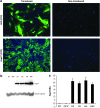
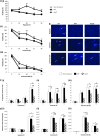
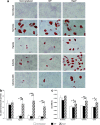
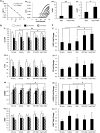
References
-
- Assmus B., et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N. Engl. J. Med. 2006;355:1222–1232. - PubMed
-
- Bruey J.M., et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell. Biol. 2000;2:645–652. - PubMed
-
- Chang W., et al. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27:2283–2292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

