Kids Safe and Smokefree (KiSS): a randomized controlled trial of a multilevel intervention to reduce secondhand tobacco smoke exposure in children
- PMID: 23987302
- PMCID: PMC3844378
- DOI: 10.1186/1471-2458-13-792
Kids Safe and Smokefree (KiSS): a randomized controlled trial of a multilevel intervention to reduce secondhand tobacco smoke exposure in children
Abstract
Background: Secondhand smoke exposure (SHSe) harms children's health, yet effective interventions to reduce child SHSe in the home and car have proven difficult to operationalize in pediatric practice. A multilevel intervention combining pediatric healthcare providers' advice with behavioral counseling and navigation to pharmacological cessation aids may improve SHSe control in pediatric populations.
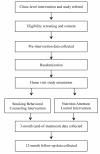
Methods/design: This trial uses a randomized, two-group design with three measurement periods: pre-intervention, end of treatment and 12-month follow-up. Smoking parents of children < 11-years-old are recruited from pediatric clinics. The clinic-level intervention includes integrating tobacco intervention guideline prompts into electronic health record screens. The prompts guide providers to ask all parents about child SHSe, advise about SHSe harms, and refer smokers to cessation resources. After receiving clinic intervention, eligible parents are randomized to receive: (a) a 3-month telephone-based behavioral counseling intervention designed to promote reduction in child SHSe, parent smoking cessation, and navigation to access nicotine replacement therapy or cessation medication or (b) an attention control nutrition education intervention. Healthcare providers and assessors are blind to group assignment. Cotinine is used to bioverify child SHSe (primary outcome) and parent quit status.
Discussion: This study tests an innovative multilevel approach to reducing child SHSe. The approach is sustainable, because clinics can easily integrate the tobacco intervention prompts related to "ask, advise, and refer" guidelines into electronic health records and refer smokers to free evidence-based behavioral counseling interventions, such as state quitlines.
Trial registration: NCT01745393 (clinicaltrials.gov).
Figures
References
-
- USDHHS. Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: US Government Printing Office; 2006. - PubMed
-
- EPA. Respiratory health effects of passive smoking: lung cancer and other disorders. Washington, D.C: U.S. Environmental Protection Agency, Office of Research and Development, Office of Health and Environmental Assessment; 1992.
-
- Metsios GS, Flouris AD, Angioi M, Koutedakis Y. Passive smoking and the development of cardiovascular disease in children: A systematic review. Cardiol Res Pract. 2011;2011 http://dx.doi.org/10.4061/2011/587650. - DOI - PMC - PubMed
-
- Twardella D, Bolte G, Fromme H, Wildner M, von Kries R. Exposure to secondhand tobacco smoke and child behaviour - results from a cross-sectional study among preschool children in Bavaria. Acta Paediatr. 2010;99(1):106–111. - PubMed
-
- American Academy of Pediatrics. Counseling parents about smoking cessation. Amerian Academy of Pediarics Resources for Clinicians. 2011. http://www.aap.org/richmondcenter/counselingParents.html.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical