Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast
- PMID: 23987569
- PMCID: PMC3766054
- DOI: 10.1186/1754-6834-6-125
Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast
Abstract
Background: Redox-cofactor balancing constrains product yields in anaerobic fermentation processes. This challenge is exemplified by the formation of glycerol as major by-product in yeast-based bioethanol production, which is a direct consequence of the need to reoxidize excess NADH and causes a loss of conversion efficiency. Enabling the use of CO2 as electron acceptor for NADH oxidation in heterotrophic microorganisms would increase product yields in industrial biotechnology.
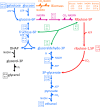
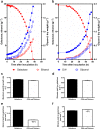
Results: A hitherto unexplored strategy to address this redox challenge is the functional expression in yeast of enzymes from autotrophs, thereby enabling the use of CO2 as electron acceptor for NADH reoxidation. Functional expression of the Calvin cycle enzymes phosphoribulokinase (PRK) and ribulose-1,5-bisphosphate carboxylase (Rubisco) in Saccharomyces cerevisiae led to a 90% reduction of the by-product glycerol and a 10% increase in ethanol production in sugar-limited chemostat cultures on a mixture of glucose and galactose. Co-expression of the Escherichia coli chaperones GroEL and GroES was key to successful expression of CbbM, a form-II Rubisco from the chemolithoautotrophic bacterium Thiobacillus denitrificans in yeast.
Conclusions: Our results demonstrate functional expression of Rubisco in a heterotrophic eukaryote and demonstrate how incorporation of CO2 as a co-substrate in metabolic engineering of heterotrophic industrial microorganisms can be used to improve product yields. Rapid advances in molecular biology should allow for rapid insertion of this 4-gene expression cassette in industrial yeast strains to improve production, not only of 1st and 2nd generation ethanol production, but also of other renewable fuels or chemicals.
Figures


References
-
- World Fuel Ethanol Production. http://ethanolrfa.org/pages/World-Fuel-Ethanol-Production.
-
- van Dijken JP, Scheffers WA. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol Rev. 1986;32:199–224.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

