Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study
- PMID: 23988361
- PMCID: PMC3758969
- DOI: 10.1136/bmjopen-2013-002984
Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study
Abstract
Objectives: To investigate whether additional catheter-directed thrombolysis (CDT) improves long-term quality of life (QOL) compared with standard treatment with anticoagulation and compression stockings alone in patients with proximal deep vein thrombosis (DVT).
Design: Open-label randomised controlled trial.
Setting: 19 Hospitals in the Norwegian southeastern health region.
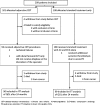
Participants: Patients (18-75 years) with a high proximal DVT, symptoms <21 days and no increased risk of bleeding were eligible. 189 of 209 recruited patients completed 24 months of follow-up.
Interventions: Participants were randomised to additional CDT with alteplase for 1-4 days or to standard treatment only with 6 months of anticoagulation and 24 months of compression stockings.
Primary and secondary outcome measures: Planned secondary outcome measures included QOL as assessed with the generic instrument EQ-5D and the disease-specific instrument VEINES-QOL/Sym. Primary outcome measure was post-thrombotic syndrome (PTS) after 24 months.
Results: After 24 months there were no differences in QOL between the additional CDT and standard treatment arms; mean difference for the EQ-5D index was 0.04 (95% CI -0.10 to 0.17), for the VEINES-QOL score 0.2 (95% CI -2.8 to 3.0) and for the VEINES-Sym score 0.5 (95% CI -2.4 to 3.4; p values>0.37). Independent of treatment arms, patients with PTS had poorer outcomes than patient without PTS; mean difference for EQ-5D was 0.09 (95% CI 0.03 to 0.15), for VEINES-QOL score 8.6 (95% CI 5.9 to 11.2) and for VEINES-Sym score 9.8 (95% CI 7.3 to 12.3; p values<0.001).
Conclusions: QOL did not differ between patients treated with additional CDT compared with standard treatment alone. Patients who developed PTS reported poorer QOL and more symptoms than patients without PTS. QOL should be included as an outcome measure in clinical studies on patients at risk of PTS.
Trial registration: NCT00251771.
References
-
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004;141:249–56 - PubMed
-
- Brandjes DP, Buller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997;349:759–62 - PubMed
-
- Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379:31–8 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical