Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation
- PMID: 23989005
- PMCID: PMC3798734
- DOI: 10.1152/ajpgi.00071.2013
Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation
Abstract
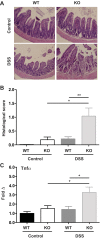
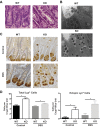
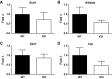
Crohn's disease (CD) is a chronic, immune-mediated, inflammatory disorder of the intestine that has been linked to numerous susceptibility genes, including the immunity-related GTPase (IRG) M (IRGM). IRGs comprise a family of proteins known to confer resistance to intracellular infections through various mechanisms, including regulation of phagosome processing, cell motility, and autophagy. However, despite its association with CD, the role of IRGM and other IRGs in regulating intestinal inflammation is unclear. We investigated the involvement of Irgm1, an ortholog of IRGM, in the genesis of murine intestinal inflammation. After dextran sodium sulfate exposure, Irgm1-deficient [Irgm1 knockout (KO)] mice showed increased acute inflammation in the colon and ileum, with worsened clinical responses. Marked alterations of Paneth cell location and granule morphology were present in Irgm1 KO mice, even without dextran sodium sulfate exposure, and were associated with impaired mitophagy and autophagy in Irgm1 KO intestinal cells (including Paneth cells). This was manifested by frequent tubular and swollen mitochondria and increased LC3-positive autophagic structures. Interestingly, these LC3-positive structures often contained Paneth cell granules. These results suggest that Irgm1 modulates acute inflammatory responses in the mouse intestine, putatively through the regulation of gut autophagic processes, that may be pivotal for proper Paneth cell functioning.
Keywords: autophagy; experimental colitis; immunity-related GTPases.
Figures




References
-
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118, 2000 - PubMed
-
- Bafica A, Feng CG, Santiago HC, Aliberti J, Cheever A, Thomas KE, Taylor GA, Vogel SN, Sher A. The IFN-inducible GTPase LRG47 (Irgm1) negatively regulates TLR4-triggered proinflammatory cytokine production and prevents endotoxemia. J Immunol 179: 5514–5522, 2007 - PubMed
-
- Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9: 356–368, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

