Crystallization of the HigBA2 toxin-antitoxin complex from Vibrio cholerae
- PMID: 23989162
- PMCID: PMC3758162
- DOI: 10.1107/S1744309113021490
Crystallization of the HigBA2 toxin-antitoxin complex from Vibrio cholerae
Abstract
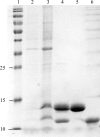
The genome of Vibrio cholerae encodes two higBA toxin-antitoxin (TA) modules that are activated by amino-acid starvation. Here, the TA complex of the second module, higBA2, as well as the C-terminal domain of the corresponding HigA2 antitoxin, have been purified and crystallized. The HigBA2 complex crystallized in two crystal forms. Crystals of form I belonged to space group P2(1)2(1)2, with unit-cell parameters a = 129.0, b = 119.8, c = 33.4 Å, and diffracted to 3.0 Å resolution. The asymmetric unit is likely to contain a single complex consisting of two toxin monomers and one antitoxin dimer. The second crystal form crystallized in space group P3(2)21, with unit-cell parameters a = 134.5, c = 55.4 Å. These crystals diffracted to 2.2 Å resolution and probably contain a complex with a different stoichiometry. Crystals of the C-terminal domain of HigA2 belonged to space group C2, with unit-cell parameters a = 115.4, b = 61.2, c = 73.8 Å, β = 106.7°, and diffracted to 1.8 Å resolution.
Keywords: intrinsic disorder; macromolecular complexes; persistence; ribonucleases; toxin–antitoxin modules.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

