50 years of hurdles and hope in anxiolytic drug discovery
- PMID: 23989795
- PMCID: PMC4176700
- DOI: 10.1038/nrd4075
50 years of hurdles and hope in anxiolytic drug discovery
Abstract
Anxiety disorders are the most prevalent group of psychiatric diseases, and have high personal and societal costs. The search for novel pharmacological treatments for these conditions is driven by the growing medical need to improve on the effectiveness and the side effect profile of existing drugs. A huge volume of data has been generated by anxiolytic drug discovery studies, which has led to the progression of numerous new molecules into clinical trials. However, the clinical outcome of these efforts has been disappointing, as promising results with novel agents in rodent studies have very rarely translated into effectiveness in humans. Here, we analyse the major trends from preclinical studies over the past 50 years conducted in the search for new drugs beyond those that target the prototypical anxiety-associated GABA (γ-aminobutyric acid)-benzodiazepine system, which have focused most intensively on the serotonin, neuropeptide, glutamate and endocannabinoid systems. We highlight various key issues that may have hampered progress in the field, and offer recommendations for how anxiolytic drug discovery can be more effective in the future.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


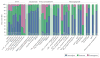
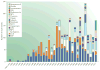


References
-
- Wittchen HU, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. This is a major landmark study that sheds new light on the state of Europe’s mental and neurological health. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

