Novel paradigms for dialysis vascular access: upstream hemodynamics and vascular remodeling in dialysis access stenosis
- PMID: 23990161
- PMCID: PMC3848396
- DOI: 10.2215/CJN.03450413
Novel paradigms for dialysis vascular access: upstream hemodynamics and vascular remodeling in dialysis access stenosis
Abstract
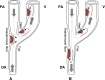
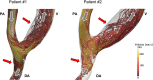
Failure of hemodialysis access is caused mostly by venous intimal hyperplasia, a fibro-muscular thickening of the vessel wall. The pathogenesis of venous neointimal hyperplasia in primary arteriovenous fistulae consists of processes that have been identified as upstream and downstream events. Upstream events are the initial events producing injury of the endothelial layer (surgical trauma, hemodynamic shear stress, vessel wall injury due to needle punctures, etc.). Downstream events are the responses of the vascular wall at the endothelial injury that consist of a cascade of processes including leukocyte adhesion, migration of smooth muscle cells from the media to the intimal layer, and proliferation. In arteriovenous fistulae, the stenoses occur in specific sites, consistently related to the local hemodynamics determined by the vessel geometry and blood flow pattern. Recent findings that the localization of these sites matches areas of disturbed flow may add new insights into the pathogenesis of neointimal hyperplasia in the venous side of vascular access after the creation of the anastomosis. The detailed study of fluid flow motion acting on the vascular wall in anastomosed vessels and in the arm vasculature at the patient-specific level may help to elucidate the role of hemodynamics in vascular remodeling and neointimal hyperplasia formation. These computational approaches may also help in surgical planning for the amelioration of clinical outcome. This review aims to discuss the role of the disturbed flow condition in acting as upstream event in the pathogenesis of venous intimal hyperplasia and in producing subsequent local vascular remodeling in autogenous arteriovenous fistulae used for hemodialysis access. The potential use of blood flow analysis in the management of vascular access is also discussed.
Figures
References
-
- Brescia MJ, Cimino JE, Appel K, Hurwich BJ: Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med 275: 1089–1092, 1966 - PubMed
-
- Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 62: 1109–1124, 2002 - PubMed
-
- Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

