Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease
- PMID: 23990350
- PMCID: PMC6486274
- DOI: 10.1002/14651858.CD006826.pub2
Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease
Abstract
Background: Both long-acting beta(2)-agonists and inhaled corticosteroids have been recommended in guidelines for the treatment of chronic obstructive pulmonary disease (COPD). Their co-administration in a combined inhaler is intended to facilitate adherence to medication regimens and to improve efficacy. Three preparations are currently available: fluticasone propionate/salmeterol (FPS). budesonide/formoterol (BDF) and mometasone furoate/formoterol (MF/F).
Objectives: To assess the efficacy and safety of combined long-acting beta2-agonist and inhaled corticosteroid (LABA/ICS) preparations, as measured by clinical endpoints and pulmonary function testing, compared with inhaled corticosteroids (ICS) alone, in the treatment of adults with chronic obstructive pulmonary disease (COPD).
Search methods: We searched the Cochrane Airways Group Specialised Register of trials, which is compiled from systematic searches of multiple literature databases. The search was conducted in June 2013. In addition, we checked the reference lists of included studies and contacted the relevant manufacturers.
Selection criteria: Studies were included if they were randomised and double-blind. Compared studies combined LABA/ICS with the ICS component.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. The primary outcomes were exacerbations, mortality and pneumonia. Health-related quality of life (as measured by validated scales), lung function and side effects were secondary outcomes. Dichotomous data were analysed as fixed-effect odds ratios with 95% confidence intervals (CIs), and continuous data as mean differences or rate ratios and 95% CIs.
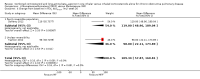
Main results: A total of 15 studies of good methodological quality met the inclusion criteria by randomly assigning 7814 participants with predominantly poorly reversible, severe COPD. Data were most plentiful for the FPS combination. Exacerbation rates were significantly reduced with combination therapies (rate ratio 0.87, 95% CI 0.80 to 0.94, 6 studies, N = 5601) compared with ICS alone. The mean exacerbation rate in the control (ICS) arms of the six included studies was 1.21 exacerbations per participant per year (range 0.88 to 1.60), and we would expect this to be reduced to a rate of 1.05 (95% CI 0.97 to 1.14) among those given combination therapy. Mortality was also lower with the combination (odds ratio (OR) 0.78, 95% CI 0.64 to 0.94, 12 studies, N = 7518) than with ICS alone, but this was heavily weighted by a three-year study of FPS. When this study was removed, no significant mortality difference was noted. The reduction in exacerbations did not translate into significantly reduced rates of hospitalisation due to COPD exacerbation (OR 0.93, 95% CI 0.80 to 1.07, 10 studies, N = 7060). Lung function data favoured combination treatment in the FPS, BDF and MF/F trials, but the improvement was small. Small improvements in health-related quality of life were measured on the St George's Respiratory Questionnaire (SGRQ) with FPS or BDF compared with ICS, but this was well below the minimum clinically important difference. Adverse event profiles were similar between the two treatments arms, and rates of pneumonia when it was diagnosed by chest x-ray (CXR) were lower than those reported in earlier trials.
Authors' conclusions: Combination ICS and LABA offer some clinical benefits in COPD compared with ICS alone, especially for reduction in exacerbations. This review does not support the use of ICS alone when LABAs are available. Adverse events were not significantly different between treatments. Further long-term assessments using practical outcomes of current and new 24-hour LABAs will help determine their efficacy and safety. For robust comparisons as to their relative effects, long-term head-to-head comparisons are needed.
Conflict of interest statement
The review authors who have been involved in this review have done so with no known conflicts of interest. None of the authors is considered a paid consultant by any pharmaceutical company that produces agents discussed in this review.
Figures


































































Update of
-
Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD006826. doi: 10.1002/14651858.CD006826. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2013 Aug 30;(8):CD006826. doi: 10.1002/14651858.CD006826.pub2. PMID: 17943917 Free PMC article. Updated.
References
References to studies included in this review
Bourbeau 2007 {published data only}
Calverley 2003 {published and unpublished data}
-
- AstraZeneca SD. A placebo‐controlled 12‐month efficacy study of the fixed combination budesonide/formoterol compared to budesonide and formoterol as monotherapies in patients with chronic obstructive pulmonary disease (COPD). AstraZeneca Clinical Trials 2002.
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference; May 21‐26; 2004, Orlando, Florida. C22 [Poster 505].
-
- Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. - PubMed
-
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P436. - PubMed
-
- Calverley PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P1587.
Doherty 2012 {published data only}
-
- Doherty DE, Kerwin E, Tashkin DP, Matiz‐Bueno CE, Shekar T, Banerjee S, et al. Combined mometasone furoate and formoterol in patients with moderate to very severe chronic obstructive pulmonary disease (COPD): phase 3 efficacy and safety study [Abstract]. Journal of Allergy and Clinical Immunology 2012; Vol. 129, issue 2 Suppl:AB75 [283].
-
- Doherty DE, Tashkin DP, Kerwin E, Knorr BA, Shekar T, Banerjee S, et al. Effects of mometasone furoate/formoterol fumarate fixed‐dose combination formulation on chronic obstructive pulmonary disease (COPD): results from a 52‐week Phase III trial in subjects with moderate‐to‐very severe COPD. International Journal of Chronic Obstructive Pulmonary Disease 2012; Vol. 7, issue 1178‐2005 (Electronic). 1176‐9106 (Linking):57‐71. - PMC - PubMed
-
- NCT00383721. A Randomized, 26‐Week, Placebo‐Controlled Efficacy and Safety Study With a 26‐Week Long‐Term Safety Extension, of High‐ and Medium‐Dose Inhaled Mometasone Furoate/Formoterol Fixed‐Dose Combination Formulation Compared With Formoterol and High‐Dose Inhaled Mometasone Furoate Monotherapy in Subjects With Moderate to Severe COPD, 2006. http://clinicaltrials.gov/show/NCT00383721. []
Hanania 2003 {published and unpublished data}
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 micro g)/salmeterol (50 micro g) combined in the diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. - PubMed
-
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the diskus. American Journal of Respiratory & Critical Care Medicine 2001;163(5 Suppl):A279.
-
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P434.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. National COPD Conference; November 14‐15, 2003; Arlington, Virginia. Abstract 1081.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to salmeterol/fluticasone propionate combination therapy in COPD [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P429.
Lapperre 2009 {published data only}
-
- Lapperre TS, Snoeck‐Stroband JB, Gosman MM, Jansen DF, Schadewijk A, Thiadens HA, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2009; Vol. 151, issue 8:517‐27. - PubMed
Mahler 2002 {published and unpublished data}
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD), 2003. http://www.abstracts2view.com.
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory Critical Care Medicine 2002;166(8):1084‐91. - PubMed
-
- SFCA3006. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 500mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
-
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal 2003;22 Suppl 45:51s.
-
- Spencer MD, Anderson JA. Salmeterol/fluticasone combination produces clinically important benefits in dyspnea and fatigue [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25, 2005; San Diego, California. B93 [Poster 308].
NCT00358358 {published data only}
-
- NCT00358358. Trial SCO104925. See detailed description, 2006. http://clinicaltrials.gov/show/NCT00358358. []
SFCT01 {unpublished data only}
-
- SFCT01. A Multicentre, Randomised, Double‐Blind, Parallel Group, Placebo‐Controlled Study to Compare the Efficacy and Safety of Inhaled Salmeterol/Fluticasone Propionate Combination Product 25/250 µg Two Puffs Bd and Fluticasone Propionate 250µg Two Puffs Bd Alone, All Administered Via Metered Dose Inhalers (MDI), in the Treatment of Subjects with Chronic Obstructive Pulmonary Disease (COPD) for 52 Weeks. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Sin 2008 {published data only}
-
- NCT00120978. Advair-CRP Study. http://clinicaltrials.gov/show/NCT00120978 2004. []
-
- Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, et al. Can inhaled fluticasone alone or in combination with salmeterol reduce systemic inflammation in chronic obstructive pulmonary disease? Study protocol for a randomized controlled trial [NCT00120978]. BMC Pulmonary Medicine 2006;6(6):3. - PMC - PubMed
-
- Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, et al. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2008; Vol. 177, issue 11:1207‐14. - PubMed
Szafranski 2003 {published and unpublished data}
-
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD [Abstract]. Thorax 2002;57(Suppl III):iii43.
-
- AstraZeneca SD. A placebo‐controlled 12 month efficacy study of the fixed combination budesonide/formoterol compared with budesonide and formoterol as monotherapies in patients with chronic obstructive pulmonary disease (COPD). AstraZeneca Clinical Trials 2001.
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference; May 21‐26, 2004; Orlando, Florida. C22 [Poster 505].
-
- Calverley P, Pauwels R, Lofdahl CG, Svensson K, Higenbottam T, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. European Respiratory Journal 2005;26(3):406‐13. - PubMed
-
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax. BTS Winter Meeting 2002:S145.
Tashkin 2008 {published data only}
-
- Astrazeneca (D5899C00002). A 6‐month double‐blind, double‐dummy, randomized, parallel group, multicenter efficacy & safety study of Symbicort® pMDI 2 × 160/4.5mg & 80/4.5mg bid compared to Formoterol TBH, Budesonide pMDI (& the combination) & placebo in COPD patients. www.astrazenecaclinicaltrials.com (accessed 8 April 2008).
-
- Tashkin DP, Rennard SI, Martin P, Goldman M, Silkoff PE. Efficacy of budesonide/formoterol administered via one pressurized metered‐dose inhaler over 6 months in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2008; Vol. 134, issue 4:105001s.
-
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease : results of a 6‐month randomized clinical trial. Drugs 2008; Vol. 68, issue 0012‐6667 (Print):1975‐2000. - PubMed
Tashkin 2012 {published data only}
-
- Kerwin E, Tashkin DP, Matiz‐Bueno CE, Doherty DE, Shekar T, Banerjee S, et al. Clinical efficacy and safety of combined mometasone furoate and formoterol in patients with moderate to very severe chronic obstructive pulmonary disease (COPD) [Abstract]. Journal of Allergy and Clinical Immunology 2012; Vol. 129, issue 2 Suppl:AB201 [759].
-
- NCT00383435. A Randomized, 26‐Week, Placebo‐Controlled Efficacy and Safety Study With a 26‐Week Long‐Term Safety Extension, of High‐ and Medium‐Dose Inhaled Mometasone Furoate/Formoterol Fixed‐Dose Combination Formulation Compared With Formoterol and High‐Dose Inhaled Mometasone Furoate Monotherapy in Subjects With Moderate to Severe COPD, 2006. http://clinicaltrials.gov/show/NCT00383435. []
-
- Tashkin DP, Doherty DE, Kerwin E, Matiz‐Bueno CE, Knorr B, Shekar T, et al. Efficacy and safety of a fixed‐dose combination of mometasone furoate and formoterol fumarate in subjects with moderate to very severe COPD: results from a 52‐week Phase III trial. International Journal of Chronic Obstructive Pulmonary Disease 2012; Vol. 7, issue 1178‐2005 (Electronic). 1176‐9106 (Linking):43‐55. - PMC - PubMed
TORCH {published and unpublished data}
-
- Briggs AH, Glick HA, Lozano‐Ortega G, Spencer M, Calverley PM, Jones PW, et al. Is treatment with ICS and LABA cost‐effective for COPD? Multinational economic analysis of the TORCH study. European Respiratory Journal 2010; Vol. 35, issue 3:532‐9. - PubMed
-
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease.[see comment]. New England Journal of Medicine 2007; Vol. 356, issue 8:775‐89. - PubMed
-
- Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Cardiovascular events in patients with chronic obstructive pulmonary disease: TORCH study results. Thorax 2010;65:719‐25. - PubMed
-
- Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. - PubMed
-
- Calverley PMA, Celli B, Ferguson G, Jenkins C, Jones PW, Pride NB, et al. Baseline characteristics of the first 5,000 COPD patients enrolled in the TORCH survival study. European Respiratory Journal 2003;22 Suppl 45:578s.
TRISTAN {published and unpublished data}
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. - PubMed
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD, 2003. http://www.abstracts2view.com A035 [Poster D50].
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/Fluticasone propionate combination for one year provides greater clinical benefit than its individual components. Proceedings of the 98th International American Thoracic Society Conference http://www.abstracts‐on‐line.com/abstracts/ATS; May 17‐22, 2002; Atlanta, Georgia. A98 [Poster 306].
-
- Calverly PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20 Suppl 38:242 [P1572].
-
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]. American Thoracic Society 100th International Conference; May 21‐26, 2004; Orlando, Florida. D22 [Poster 503].
Zhong 2012 {published data only}
-
- Zhong N, Zheng J, Wen F, Yang L, Chen P, Xiu Q, et al. Efficacy and safety of budesonide/formoterol via a dry powder inhaler in Chinese patients with chronic obstructive pulmonary disease. Current Medical Research and Opinion 2012; Vol. 28, issue 2:257‐65. - PubMed
-
- Zhong N, Zheng J, Wen F, Yang L, Chen P, Xiu Q, et al. Efficacy and safety of inhalation of budesonide/formoterol via turbuhaler in Chinese patients with chronic obstructive pulmonary disease [Abstract]. Chest 2011; Vol. 140, issue 4:5225A.
References to studies excluded from this review
Aaron 2007 {published data only}
-
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone‐salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial.[see comment]. Annals of Internal Medicine 2007; Vol. 146, issue 8:545‐55. - PubMed
Bathoorn 2008 {published data only}
-
- Bathoorn E, Liesker JJ, Postma DS, Boorsma M, Bondesson E, Koëter GH, et al. Anti‐inflammatory effects of combined budesonide/formoterol in COPD exacerbations. COPD 2008; Vol. 5, issue 5:282‐90. - PubMed
Bleecker 2011 {published data only}
-
- Bleecker ER, Meyers DA, Bailey WC, Sims AM, Bujac SR, Goldman M, et al. Effect of β2‐adrenergic receptor gene polymorphism Gly16Arg on response to budesonide/formoterol pressurized metered‐dose inhaler In chronic obstructive pulmonary disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011; Vol. 183, issue Meeting Abstracts:A4086.
Calverley 2005 {published data only}
-
- Calverley PM, Szafranski W, Andersson A. Budesonide/formoterol is a well‐tolerated long term maintenance therapy for COPD [Abstract]. European Respiratory Journal 2005; Vol. 26 Suppl 49:Abstract No. 1917.
Cukier 2007 {published data only}
-
- Cukier A, Ferreira CAS, Stelmach R, Ribeiro M, Cortopassi F, Calverley PMA. The effect of bronchodilators and oxygen alone and in combination on self‐paced exercise performance in stable COPD. Respiratory Medicine 2007;101(4):743‐53. - PubMed
D5899C00001 {published data only}
-
- A 12‐Month Double‐blind, Double‐dummy, Randomized, Parallel‐group, Multicenter Efficacy & Safety Study of SYMBICORT® pMDI 2 × 160/4.5 mcg bid and 2 × 80/4.5mcg bid Compared to Formoterol TBH 2 × 4.5 mcg bid and Placebo in Patients with COPD. AstraZeneca 2008.
De Backer 2011 {published data only}
-
- Backer L, Backer J, Vos W, Holsbeke C, Vinchurkar S, Backer W. Double blind, placebo controlled crossover study in COPD patients to assess the acute effect of budesonide/formoterol using HRCT and lung function tests [Abstract]. European Respiratory Society Annual Congress; September 24‐28, 2011; Amsterdam, The Netherlands Vol. 38, issue 55:600s [P3362].
Ferguson 2006 {published data only}
-
- Ferguson GT. Cardiovascular safety of simultaneous therapy with Advair and Combivent in the treatment of COPD [Abstract]. Proceedings of the American Thoracic Society; June 15, 2006; San Diego, California A109 [Poster J2].
GlaxoSmithKline 2004 {published data only}
-
- GlaxoSmithKline SAS40007. A Randomised, Double‐Blind, Double‐Dummy, Parallel‐Group Comparison of SERETIDE DISKUS/ACCUHALER (50/100 µg Strength) twice daily (bid) with Budesonide 400 µg bid in Adolescents and Adults with Reversible Airways Obstruction. http://ctr.gsk.co.uk/Summary/fluticasone_salmeterol/IV_SAS40007.pdf. GlaxoSmithKline Clinical Trial Register, 2004.
GlaxoSmithKline 2004a {published data only}
-
- GlaxoSmithKline SAS40006. A Randomised, Double‐Blind, Double‐Dummy, Parallel‐Group Comparison of Seretide DISKUS/ACCUHALER (50/250g Strength) b.i.d. with Budesonide 800g b.i.d. in Adolescents and Adults with Reversible Airways Obstruction. http://ctr.gsk.co.uk/Summary/fluticasone_salmeterol/IV_SAS40006.pdf. GlaxoSmithKline Clinical Trial Register, 2004.
GlaxoSmithKline 2006 {published data only}
-
- GlaxoSmithKline SCO30005. A 13‐Week, Double‐Blind, Parallel‐Group, Multicentre Study to Compare the Bronchial Anti‐inflammatory Activity of the Combination of Salmeterol/ Fluticasone Propionate (SERETIDE™/ADVAIR™/VIANI™) 50/500 mcg Twice Daily Compared With Placebo Twice Daily in Patients With Chronic Obstructive Pulmonary Disease. GlaxoSmithKline Clinical Trial Register, 2006.
Golabi 2006 {published data only}
-
- Golabi P, Topaloglu N, Karakurt S, Celikel T. Effects of tiotropium and salmeterol/fluticasone combination on lung hyperinflation dyspnea and exercise tolerance in COPD [Abstract]. European Respiratory Journal 2006;28(Suppl 50):33s.
Haque 2006 {published data only}
-
- Haque RA, Torrego A, Essilfie‐Quaye S, Kharitonov SA, Johnson M, Adcock IM, et al. Effect of salmeterol and fluticasone on glucocorticoid receptor translocation in sputum macrophages and peripheral blood mononuclear cells from patients with chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society; May 23‐26, 2006; San Diego, California. A848.
INSPIRE {published data only}
-
- GlaxoSmithKline (SCO40036). Multicentre, randomised, double‐blind, double‐dummy, parallel group, 104‐week study to compare the effect of the salmeterol/fluticasone propionate combination product (SERETIDE*) 50/500mcg delivered twice daily via the DISKUS*/ACCUHALER* inhaler with tiotropium bromide 18 mcg delivered once daily via the HandiHaler inhalation device on the rate of health care utilisation exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD). http://ctr.gsk.co.uk (accessed 8 April 2008).
-
- Seemungal T, Stockley R, Calverley P, Hagan G, Wedzicha JA. Investigating new standards for prophylaxis in reduction of exacerbations-the INSPIRE study methodology. Journal of Chronic Obstructive Pulmonary Disease 2007;4(3):177‐83. - PubMed
-
- Wedzicha J, Stockley R, Seemungal T, Hagan G, Calverley P. The INSPIRE study: effect of salmeterol/fluticasone propionate versus tiotropium bromide on COPD exacerbations. Respirology 2007;12(Suppl 4):A112.
Jiang 2011 {published data only}
-
- Jiang YP, Zhao YF, Yang Y. Effect of seretide on quality of life in COPD: measured with COPD assessment test [Abstract]. Respirology (Carlton, Vic.) 2011; Vol. 16, issue Suppl 2:100 [344].
Jung 2012 {published data only}
-
- Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respiratory Medicine 2012; Vol. 106, issue 3:382‐9. - PubMed
Laties 2010 {published data only}
-
- Laties A, Rennard SI, Tashkin DP, Suchower LJ, Martin UJ. Effect of budesonide/formoterol pressurized metered‐dose inhaler (bud/fm pmdi) on ophthalmologic assessments in moderate to very severe chronic obstructive pulmonary disease (COPD) patients: results from a 1‐year, randomized, controlled clinical trial [Abstract]. Chest 2010; Vol. 138, issue 4:468A.
Lindberg 2007 {published data only}
-
- Lindberg A, Szalai Z, Pullerits T, Radeczky E. Fast onset of effect of budesonide/formoterol versus salmeterol/fluticasone and salbutamol in patients with chronic obstructive pulmonary disease and reversible airway obstruction. Respirology (Carlton, Vic.) 2007; Vol. 12, issue 5:732‐9. - PubMed
Mittmann 2010 {published data only}
-
- Mittmann N, Hernandez P, Mellsträm C, Brannman L, Welte T, Mellström C, et al. Cost‐effectiveness of budesonide/formoterol added to tiotropium in COPD patients in Canada, Australia and Sweden [Abstract]. European Respiratory Society Annual Congress; September 18‐22, 2010; Barcelona, Spain [5183].
Mittmann 2011 {published data only}
-
- Mittmann N, Hernandez P, Mellstrom C, Brannman L, Welte T. Cost effectiveness of budesonide/formoterol added to tiotropium bromide versus placebo added to tiotropium bromide in patients with chronic obstructive pulmonary disease: Australian, Canadian and Swedish healthcare perspectives. PharmacoEconomics 2011; Vol. 29, issue 5:403‐14. - PubMed
NCT00269126 {published data only}
-
- NCT00269126. See detailed description, 2005. http://clinicaltrials.gov/show/NCT00269126. []
NCT00476099 {published data only}
-
- NCT00476099. A 48‐Week, Double Blind, Double Dummy, Randomised, Multinational, Multicentre, 3‐Arm Parallel Group Clinical Study of "Fixed Combination" Beclometasone Dipropionate Plus Formoterol Fumarate Administered Via pMDI With HFA‐134a Propellant Versus "Fixed Combination" Budesonide Plus Formoterol DPI Versus Formoterol DPI in Patients With Stable Severe Chronic Obstructive Pulmonary Disease (COPD), 2006. http://clinicaltrials.gov/show/NCT00476099. []
NCT00549146 {published data only}
-
- NCT00549146. Trial: SCO40055, 2003. http://clinicaltrials.gov/show/NCT00549146. []
Rennard 2008 {published data only}
-
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Silkoff PE. Long‐term tolerability of budesonide and formoterol administered in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2008; Vol. 134, issue 4:103003s.
Rennard 2009 {published data only}
-
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered‐dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1‐year randomized controlled clinical trial. Drugs. Adis Data Information BV., 2009; Vol. 69, issue 0012‐6667:549‐65. - PMC - PubMed
Rennard 2009a {published data only}
-
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered‐dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1‐year randomized controlled clinical trial. Drugs 2009;69:549‐65. - PMC - PubMed
Rennard 2010 {published data only}
-
- Rennard SI, Tashkin DP, Suchower LJ, Martin UJ. Effect of budesonide/formoterol pressurized metered‐dose inhaler (BUD/FM pMDI) on bone mineral density (BMD) in moderate to very severe chronic obstructive pulmonary disease (COPD) patients: results from a 1‐year, randomized, controlled clinical trial [Abstract]. Chest 2010; Vol. 138, issue 4:863A.
Sagcan 2007 {published data only}
-
- Sagcan G, Memis U, Cuhadaroglu C, Sen C, Duygu E. The effects of formoterol/budesonide on sleep quality of COPD patients [Abstract]. European Respiratory Journal 2007; Vol. 30, issue Suppl 51:507s [E3049].
Schermer 2007 {published data only}
-
- Schermer TR, Albers JM, Verblackt HW, Costongs RJ, Westers P. Lower inhaled steroid requirement with a fluticasone/salmeterol combination in family practice patients with asthma or COPD. Family Practice 2007; Vol. 24, issue 2:181‐8. - PubMed
SCO100250 {unpublished data only}
-
- GlaxoSmithKline (SCO100250). A randomized, double‐blind, parallel‐group, 52‐week study to compare the effect of fluticasone propionate/salmeterol DISKUS 250/50mcg bid with salmeterol DISKUS 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD). http://ctr.gsk.co.uk (accessed 8 April 2008).
SCO40043 {unpublished data only}
-
- SCO40043. A randomized, double‐blind, parallel‐group, 52‐week study to compare the effect of fluticasone propionate/salmeterol DISKUS® 250/50mcg bid with salmeterol DISKUS® 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD). http://ctr.gsk.co.uk (accessed 8 April 2008).
Sethi 2006 {published data only}
-
- Sethi S, Grove L, Wrona C, Maloney J. Prevalence of bacterial colonization in COPD is not altered by fluticasone/salmeterol. Proceedings of the American Thoracic Society; May 23‐26. 2006; San Diego, California. A115.
Shaker 2009 {published data only}
-
- Shaker SB, Dirksen A, Ulrik CS, Hestad M, Stavngaard T, Laursen LC, et al. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD 2009; Vol. 6, issue 2:104‐11. - PubMed
Sharafkhaneh 2011 {published data only}
-
- Sharafkhaneh A, Southard J, Goldman M, Uryniak T, Martin UJ. Long‐term effect of budesonide/formoterol pressurized metered‐dose inhaler on exacerbations and pulmonary function in patients with chronic obstructive pulmonary disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011; Vol. 183, issue Meeting Abstracts:A1599.
Southard 2011 {published data only}
-
- Southard JG, Sharafkhaneh A, Goldman M, Uryniak T, Martin UJ. Long‐term tolerability of budesonide/formoterol pressurized metered‐dose inhaler In patients with chronic obstructive pulmonary disease and a history of exacerbations [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011; Vol. 183, issue Meeting Abstracts:A1597.
Stallberg 2008 {published data only}
-
- Stallberg B, Andersson EBC, Ekstrom T, Selroos O, Vogelmeier C, Larsson K. Budesonide /formoterol for the treatment of COPD exacerbations in the primary healthcare setting [Abstract]. European Respiratory Society Annual Congress; October 4‐8, 2008; Berlin, Germany [P3610].
Sutherland 2006 {published data only}
-
- Sutherland ER, Moss TA, Stevens AD, Pak J, Martin RJ. Modulation of sputum gene expression in COPD by fluticasone /salmeterol. European Respiratory Journal 2006;28(Suppl 50):662s.
Trofimenko 2006 {published data only}
-
- Trofimenko IN, Chernyak BA. The efficacy of salmeterol/fluticasone (SF) for 6 month's therapy at severe COPD patients. European Respiratory Journal 2006;28(Suppl 50):30s.
Welte 2009 {published data only}
-
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2009; Vol. 180, issue 8:741‐50. - PubMed
Welte 2009a {published data only}
-
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Addition of budesonide/formoterol to tiotropium reduces the number of exacerbation days compared with tiotropium alone [Abstract]. Chest 2009; Vol. 136, issue 4:26S‐f.
Welte 2009b {published data only}
-
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Budesonide/formoterol added to tiotropium provides rapid improvements in lung function and ability to undertake morning activities [Abstract]. Chest 2009; Vol. 136, issue 4:24S‐g.
Welte 2009c {published data only}
-
- Welte T, Hartman L, Polanowski T, Hernandez P, Miravitlles M, Peterson S, et al. Budesonide/formoterol added to tiotropium is well tolerated and reduces risk of severe exacerbations in COPD patients [Abstract]. American Thoracic Society International Conference; May 15‐20, 2009; San Diego, California A6188 [Poster #215].
Welte 2009d {published data only}
-
- Welte T, Miravitlles M, Hernandez P, Peterson S, Polanowski T, Kessler R, et al. Budesonide/formoterol added to tiotropium improves exacerbations and exacerbation‐related antibiotic use in patients with COPD [Abstract]. European Respiratory Society Annual Congress; September 12‐16, 2009; Vienna, Austria [P2012].
Wilson 2007 {published data only}
-
- Wilson DS, Gillion MS, Rees PJ. Use of dry powder inhalers in COPD. International Journal of Clinical Practice 2007; Vol. 61, issue 12:2005‐8. - PubMed
Worth 2009 {published data only}
-
- Worth H, Foerster K, Peterson S, Nihlen U, Magnussen H, et al. Budesonide/formoterol improves exercise tolerance compared with placebo and formoterol in COPD patients [Abstract]. European Respiratory Society Annual Congress; September 12‐16, 2009; Vienna, Austria [201].
Worth 2009a {published data only}
-
- Worth H, Peterson S, Nihlen U, Magnussen H. Improved exercise tolerance with budesonide/formoterol vs placebo and formoterol in COPD patients [Abstract]. American Thoracic Society International Conference; May 15‐20, 2009; San Diego, California A6193 [Poster #220].
Worth 2010 {published data only}
-
- Worth H, Förster K, Eriksson G, Nihlén U, Peterson S, Magnussen H. Budesonide added to formoterol contributes to improved exercise tolerance in patients with COPD. Respiratory Medicine 2010; Vol. 104, issue 10:1450‐9. - PubMed
Zheng 2006 {published data only}
-
- Zheng J, Zhong N, Yang L, Wu Y, Chen P, Wen Z, et al. The efficacy and safety of fluticasone propionate 500 mg/salmeterol 50 mg combined via diskus/accuhaler in Chinese patients with chronic obstructive pulmonary disease (COPD). Chest 2006;130(4):182s.
-
- Zhong N, Zheng J, Yang L, Wu Y, Chen P, Wen Z, et al. The efficacy and safety of salmeterol 50µg/fluticasone propionate 500µg combined via accuhaler in Chinese patients with chronic obstructive pulmonary disease [Abstract]. Respirology 2006;11(Suppl 5):A150.
Additional references
Appleton 2006
Barnes 2002
-
- Barnes PJ. Scientific rationale for inhaled combination therapy with long‐acting b2‐agonists and corticosteroids. European Respiratory Journal 2002;19:182‐91. - PubMed
Cates 2012
Cosio 2009
-
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. New England Journal of Medicine 2009;360:2445‐54. - PubMed
Dong 2013
-
- Dong YH, Lin HH, Shau WY, Wu YC, Chang CH, Lai MS. Comparative safety of inhaled medications inpatients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta‐analysis of randomised controlled trials. Thorax 2013;68(2):48‐56. - PubMed
Drummond 2008
Garcia‐Aymerich 2011
-
- Garcia‐Aymerich J, Gómez FP, Benet M, Farrero E, Basagaña X, Gayete À, et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax [published online first] 21 December 2010;66(5):430‐7. - PubMed
GOLD 2011
-
- Global Strategy for Diagnosis, Management, Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2011. http://www.goldcopd.org.
Hanania 2008
-
- Hanania NA. The impact of inhaled corticosteroid and long‐acting beta‐agonist combination therapy on outcomes in COPD. Pulmonary Pharmacology and Therapeutics 2008;21(3):540‐50. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jones 2002
-
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal 2002;19:398‐404. - PubMed
Karner 2011
Kohansal 2009
-
- Kohansal R, Martinez‐Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. American Journal of Respiratory and Critical Care Medicine 2009;180(1):3‐10. - PubMed
NCGC 2010
-
- London: National Clinical Guideline Centre. Management of chronic obstructive pulmonary disease in adults in primary and secondary care, 2010. http://guidance.nice.org.uk/CG101/Guidance/pdf/English. - PMC - PubMed
NICE 2010
-
- CG101 Chronic obstructive pulmonary disease (update): NICE guideline, 2010. http://guidance.nice.org.uk/CG101/NICEGuidance/pdf/English.
RevMan 5.2 [Computer program]
-
- The Cochrane Collaboration. Copenhagen: The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.2. The Cochrane Collaboration. Copenhagen: The Nordic Cochrane Centre, 2012.
Rodrigo 2012
-
- Rodrigo GJ. Castro‐Rodríguez JA. Safety of long‐acting beta agonists for the treatment of asthma: clearing the air. Thorax 2012 21 April 2011;67:342‐9. - PubMed
Visual Rx [Computer program]
-
- Cates CJ. Visual Rx. Version 3.0, 2008, www.nntonline.net.
References to other published versions of this review
Nannini 2003
Nannini 2004
Nannini 2007
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

