Functional characterization of human myosin-18A and its interaction with F-actin and GOLPH3
- PMID: 23990465
- PMCID: PMC3798472
- DOI: 10.1074/jbc.M113.497180
Functional characterization of human myosin-18A and its interaction with F-actin and GOLPH3
Abstract
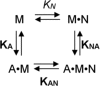
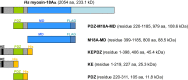
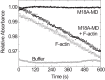
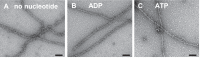
Molecular motors of the myosin superfamily share a generic motor domain region. They commonly bind actin in an ATP-sensitive manner, exhibit actin-activated ATPase activity, and generate force and movement in this interaction. Class-18 myosins form heavy chain dimers and contain protein interaction domains located at their unique N-terminal extension. Here, we characterized human myosin-18A molecular function in the interaction with nucleotides, F-actin, and its putative binding partner, the Golgi-associated phosphoprotein GOLPH3. We show that myosin-18A comprises two actin binding sites. One is located in the KE-rich region at the start of the N-terminal extension and appears to mediate ATP-independent binding to F-actin. The second actin-binding site resides in the generic motor domain and is regulated by nucleotide binding in the absence of intrinsic ATP hydrolysis competence. This core motor domain displays its highest actin affinity in the ADP state. Electron micrographs of myosin-18A motor domain-decorated F-actin filaments show a periodic binding pattern independent of the nucleotide state. We show that the PDZ module mediates direct binding of myosin-18A to GOLPH3, and this interaction in turn modulates the actin binding properties of the N-terminal extension. Thus, myosin-18A can act as an actin cross-linker with multiple regulatory modulators that targets interacting proteins or complexes to the actin-based cytoskeleton.
Keywords: Actin; Cytoskeleton; Myosin; Protein Domains; Protein-Protein Interactions.
Figures





References
-
- Furusawa T., Ikawa S., Yanai N., Obinata M. (2000) Isolation of a novel PDZ-containing myosin from hematopoietic supportive bone marrow stromal cell lines. Biochem. Biophys. Res. Commun. 270, 67–75 - PubMed
-
- Isogawa Y., Kon T., Inoue T., Ohkura R., Yamakawa H., Ohara O., Sutoh K. (2005) The N-terminal domain of MYO18A has an ATP-insensitive actin-binding site. Biochemistry 44, 6190–6196 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

