Statistical and bioanalytical considerations for establishing a depletion criterion for specificity testing during immunogenicity assessment of a biotherapeutic
- PMID: 23990502
- PMCID: PMC3787216
- DOI: 10.1208/s12248-013-9523-1
Statistical and bioanalytical considerations for establishing a depletion criterion for specificity testing during immunogenicity assessment of a biotherapeutic
Abstract
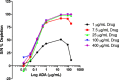
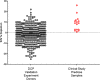
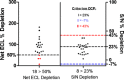
Immunogenicity assessment of fully human monoclonal antibody-based biotherapeutics requires sensitive and specific ligand binding assays. One of the components of specificity is the depletion of signal by a relevant biotherapeutic that is commonly based on an arbitrary depletion criterion of inhibition of the original response or reduction of the signal below the screening assay cut point (ACP). Hence, there is a need to develop a statistically derived physiologically relevant specificity criterion. We illustrate an optimization approach to determine the concentration of biotherapeutic required for the specificity evaluation. Naïve donor sample sets with and without circulating drug and antitherapeutic/drug antibody (ADA) were prepared. Next, a depletion cut point (DCP) using naïve and ADA-containing donor sets with the optimized biotherapeutic concentration was evaluated. A statistically derived design of experiment was used to establish a validated DCP. A reliable DCP requires naïve (no ADA) donors treated only with an optimized concentration of biotherapeutic. The additional DCPs generated using two distinct concentrations of ADA-spiked sample sets led to a physiologically irrelevant criterion that was not necessarily representative of real-time samples. This increased the risk of false positives or negatives. In this study, well-defined bioanalytical and statistical methods were employed to validate a DCP to confirm the presence of biotherapeutic specific ADA in human serum samples. A physiologically relevant and effective strategy to confirm specificity in immune reactive samples, especially those that are close to the ACP, is proposed through this study.
Figures

Similar articles
-
Recommendations for the characterization of immunogenicity response to multiple domain biotherapeutics.J Immunol Methods. 2014 Jun;408:1-12. doi: 10.1016/j.jim.2014.05.010. Epub 2014 May 24. J Immunol Methods. 2014. PMID: 24861938 Review.
-
Immunogenicity of Therapeutic Antibodies: Monitoring Antidrug Antibodies in a Clinical Context.Ther Drug Monit. 2017 Aug;39(4):327-332. doi: 10.1097/FTD.0000000000000404. Ther Drug Monit. 2017. PMID: 28463887 Review.
-
Mitigation of Pre-existing Antibodies to a Biotherapeutic in Non-clinical Species When Establishing Anti-drug Antibody Assay Cutpoint.AAPS J. 2017 Jan;19(1):313-319. doi: 10.1208/s12248-016-0011-2. Epub 2016 Nov 21. AAPS J. 2017. PMID: 27873117
-
Pre-existing Antibody: Biotherapeutic Modality-Based Review.AAPS J. 2016 Mar;18(2):311-20. doi: 10.1208/s12248-016-9878-1. Epub 2016 Jan 28. AAPS J. 2016. PMID: 26821802 Free PMC article. Review.
-
Immunogenicity testing strategy and bioanalytical assays for antibody-drug conjugates.Bioanalysis. 2013 May;5(9):1041-55. doi: 10.4155/bio.13.10. Bioanalysis. 2013. PMID: 23641695
Cited by
-
Recommendations for Systematic Statistical Computation of Immunogenicity Cut Points.AAPS J. 2017 Sep;19(5):1487-1498. doi: 10.1208/s12248-017-0107-3. Epub 2017 Jul 21. AAPS J. 2017. PMID: 28733862
References
-
- CBER UFC. Assay development for immunogenicity testing of therapeutic proteins 2009.
-
- EMEA. Guideline on immunogenicity assessment of biotechnology derived therapeutic proteins. 2007.
-
- Chamberlain P, Mire-Sluis A. An overview of scientific and regulatory issues for the immunogenicity of biological products. Dev Biol. 2003;112:3–11. - PubMed
-
- CHMP. Guideline of immunogenicity assessment of biotechnology-derived therapeutic proteins. EMEA Guidance Document. 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

