Human milk oligosaccharides protect bladder epithelial cells against uropathogenic Escherichia coli invasion and cytotoxicity
- PMID: 23990566
- PMCID: PMC3883170
- DOI: 10.1093/infdis/jit464
Human milk oligosaccharides protect bladder epithelial cells against uropathogenic Escherichia coli invasion and cytotoxicity
Abstract
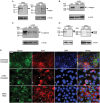
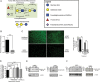
The invasive pathogen uropathogenic Escherichia coli (UPEC) is the primary cause of urinary tract infections (UTIs). Recurrent infection that can progress to life-threatening renal failure has remained as a serious global health concern in infants. UPEC adheres to and invades bladder epithelial cells to establish infection. Studies have detected the presence of human milk oligosaccharides (HMOs) in urine of breast-fed, but not formula-fed, neonates. We investigated the mechanisms HMOs deploy to elicit protection in human bladder epithelial cells infected with UPEC CFT073, a prototypic urosepsis-associated strain. We found a significant reduction in UPEC internalization into HMO-pretreated epithelial cells without observing any significant effect in UPEC binding to these cells. This event coincides with a rapid decrease in host cell cytotoxicity, recognized by LIVE/DEAD staining and cell detachment, but independent of caspase-mediated or mitochondrial-mediated programmed cell death pathways. Further investigation revealed HMOs, and particularly the sialic acid-containing fraction, reduced UPEC-mediated MAPK and NF-κB activation. Collectively, our results indicate that HMOs can protect bladder epithelial cells from deleterious cytotoxic and proinflammatory effects of UPEC infection, and may be one contributing mechanism underlying the epidemiological evidence of reduced UTI incidence in breast-fed infants.
Keywords: apoptosis; bladder epithelial cells; cell adhesion; human milk oligosaccharides; urinary tract infection; uropathogenic E. coli.
Figures



Comment in
-
Multifaceted effects of human milk oligosaccharides.J Infect Dis. 2014 Feb 1;209(3):323-4. doi: 10.1093/infdis/jit559. Epub 2013 Oct 23. J Infect Dis. 2014. PMID: 24154736 No abstract available.
References
-
- Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302–8. - PubMed
-
- Chakupurakal R, Ahmed M, Sobithadevi DN, Chinnappan S, Reynolds T. Urinary tract pathogens and resistance pattern. J Clin Pathol. 2010;63:652–4. - PubMed
-
- Catal F, Bavbek N, Bayrak O, et al. Antimicrobial resistance patterns of urinary tract pathogens and rationale for empirical therapy in Turkish children for the years 2000–2006. Int Urol Nephrol. 2009;41:953–7. - PubMed
-
- Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

