Using poly(lactic-co-glycolic acid) microspheres to encapsulate plasmid of bone morphogenetic protein 2/polyethylenimine nanoparticles to promote bone formation in vitro and in vivo
- PMID: 23990717
- PMCID: PMC3748902
- DOI: 10.2147/IJN.S45184
Using poly(lactic-co-glycolic acid) microspheres to encapsulate plasmid of bone morphogenetic protein 2/polyethylenimine nanoparticles to promote bone formation in vitro and in vivo
Abstract
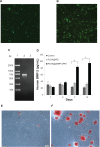
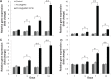
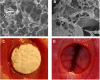
Repair of large bone defects is a major challenge, requiring sustained stimulation to continually promote bone formation locally. Bone morphogenetic protein 2 (BMP-2) plays an important role in bone development. In an attempt to overcome this difficulty of bone repair, we created a delivery system to slowly release human BMP-2 cDNA plasmid locally, efficiently transfecting local target cells and secreting functional human BMP-2 protein. For transfection, we used polyethylenimine (PEI) to create pBMP-2/PEI nanoparticles, and to ensure slow release we used poly(lactic-co-glycolic acid) (PLGA) to create microsphere encapsulated pBMP-2/PEI nanoparticles, PLGA@pBMP-2/PEI. We demonstrated that pBMP-2/PEI nanoparticles could slowly release from the PLGA@pBMP-2/PEI microspheres for a long period of time. The 3-15 μm diameter of the PLGA@pBMP-2/PEI further supported this slow release ability of the PLGA@pBMP-2/PEI. In vitro transfection assays demonstrated that pBMP-2/PEI released from PLGA@pBMP-2/PEI could efficiently transfect MC3T3-E1 cells, causing MC3T3-E1 cells to secrete human BMP-2 protein, increase calcium deposition and gene expressions of alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), SP7 and I type collagen (COLL I), and finally induce MC3T3-E1 cell differentiation. Importantly, in vivo data from micro-computed tomography (micro-CT) and histological staining demonstrated that the human BMP-2 released from PLGA@pBMP-2/PEI had a long-term effect locally and efficiently promoted bone formation in the bone defect area compared to control animals. All our data suggest that our PLGA-nanoparticle delivery system efficiently and functionally delivers the human BMP-2 cDNA and has potential clinical application in the future after further modification.
Keywords: biodegradable polymer; bone regeneration; gene therapy; human BMP-2.
Figures




Similar articles
-
Sustained release poly (lactic-co-glycolic acid) microspheres of bone morphogenetic protein 2 plasmid/calcium phosphate to promote in vitro bone formation and in vivo ectopic osteogenesis.Am J Transl Res. 2015 Dec 15;7(12):2561-72. eCollection 2015. Am J Transl Res. 2015. PMID: 26885257 Free PMC article.
-
PELA microspheres with encapsulated arginine-chitosan/pBMP-2 nanoparticles induce pBMP-2 controlled-release, transfected osteoblastic progenitor cells, and promoted osteogenic differentiation.Artif Cells Nanomed Biotechnol. 2017 Mar;45(2):330-339. doi: 10.3109/21691401.2016.1153480. Epub 2016 Mar 9. Artif Cells Nanomed Biotechnol. 2017. PMID: 26961803
-
Study of PLGA microspheres loaded with pOsx/PEI nanoparticles for repairing bone defects in vivo and in vitro.Adv Clin Exp Med. 2020 Apr;29(4):431-440. doi: 10.17219/acem/116752. Adv Clin Exp Med. 2020. PMID: 32364686
-
Long-acting PLGA microspheres: Advances in excipient and product analysis toward improved product understanding.Adv Drug Deliv Rev. 2023 Jul;198:114857. doi: 10.1016/j.addr.2023.114857. Epub 2023 May 5. Adv Drug Deliv Rev. 2023. PMID: 37149041 Review.
-
Biomedical applications of PLGA nanoparticles in nanomedicine: advances in drug delivery systems and cancer therapy.Expert Opin Drug Deliv. 2023 Jul-Dec;20(7):937-954. doi: 10.1080/17425247.2023.2223941. Epub 2023 Jun 23. Expert Opin Drug Deliv. 2023. PMID: 37294853 Review.
Cited by
-
Histochemistry for Molecular Imaging in Nanomedicine.Int J Mol Sci. 2024 Jul 24;25(15):8041. doi: 10.3390/ijms25158041. Int J Mol Sci. 2024. PMID: 39125610 Free PMC article. Review.
-
BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry.Pharmaceutics. 2019 Aug 5;11(8):393. doi: 10.3390/pharmaceutics11080393. Pharmaceutics. 2019. PMID: 31387267 Free PMC article. Review.
-
Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings.Int J Mol Sci. 2014 Jul 4;15(7):11878-921. doi: 10.3390/ijms150711878. Int J Mol Sci. 2014. PMID: 25000263 Free PMC article. Review.
-
Enhancing regenerative approaches with nanoparticles.J R Soc Interface. 2017 Apr;14(129):20170093. doi: 10.1098/rsif.2017.0093. J R Soc Interface. 2017. PMID: 28404870 Free PMC article. Review.
-
Comparative Efficiency of Gene-Activated Matrices Based on Chitosan Hydrogel and PRP Impregnated with BMP2 Polyplexes for Bone Regeneration.Int J Mol Sci. 2022 Nov 25;23(23):14720. doi: 10.3390/ijms232314720. Int J Mol Sci. 2022. PMID: 36499056 Free PMC article.
References
-
- Meyer U, Joos U, Wiesmann HP. Biological and biophysical principles in extracorporeal bone tissue engineering: part I. Int J Oral Maxillofac Surg. 2004;33:325–332. - PubMed
-
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. - PubMed
-
- Kang SW, Kim JS, Park KS, et al. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone. 2011;48:298–306. - PubMed
-
- Kirby GTS, White LJ, Rahman, et al. PLGA-Based microparticles for the sustained release of BMP-2. Polymers. 2011;3:571–586.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

