The secreted antifungal protein thionin 2.4 in Arabidopsis thaliana suppresses the toxicity of a fungal fruit body lectin from Fusarium graminearum
- PMID: 23990790
- PMCID: PMC3749967
- DOI: 10.1371/journal.ppat.1003581
The secreted antifungal protein thionin 2.4 in Arabidopsis thaliana suppresses the toxicity of a fungal fruit body lectin from Fusarium graminearum
Abstract
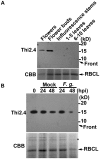
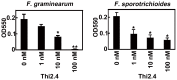
Plants possess active defense systems and can protect themselves from pathogenic invasion by secretion of a variety of small antimicrobial or antifungal proteins such as thionins. The antibacterial and antifungal properties of thionins are derived from their ability to induce open pore formation on cell membranes of phytopathogens, resulting in release of potassium and calcium ions from the cell. Wheat thionin also accumulates in the cell walls of Fusarium-inoculated plants, suggesting that it may have a role in blocking pathogen infection at the plant cell walls. Here we developed an anti-thionin 2.4 (Thi2.4) antibody and used it to show that Thi2.4 is localized in the cell walls of Arabidopsis and cell membranes of F. graminearum, when flowers are inoculated with F. graminearum. The Thi2.4 protein had an antifungal effect on F. graminearum. Next, we purified the Thi2.4 protein, conjugated it with glutathione-S-transferase (GST) and coupled the proteins to an NHS-activated column. Total protein from F. graminearum was applied to GST-Thi2.4 or Thi2.4-binding columns, and the fungal fruit body lectin (FFBL) of F. graminearum was identified as a Thi2.4-interacting protein. This interaction was confirmed by a yeast two-hybrid analysis. To investigate the biological function of FFBL, we infiltrated the lectin into Arabidopsis leaves and observed that it induced cell death in the leaves. Application of FFBL at the same time as inoculation with F. graminearum significantly enhanced the virulence of the pathogen. By contrast, FFBL-induced host cell death was effectively suppressed in transgenic plants that overexpressed Thi2.4. We found that a 15 kD Thi2.4 protein was specifically expressed in flowers and flower buds and suggest that it acts not only as an antifungal peptide, but also as a suppressor of the FFBL toxicity. Secreted thionin proteins are involved in this dual defense mechanism against pathogen invasion at the plant-pathogen interface.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Ahuja I, Kissen R, Bones AM (2012) Phytoalexins in defense against pathogens. Trends Plant Sci 17: 73–90. - PubMed
-
- Castro MS, Fontes W (2005) Plant defense and antimicrobial peptides. Protein Pept Lett 12: 13–18. - PubMed
-
- Rafiqi M, Ellis JG, Ludowici VA, Hardham AR, Dodds PN (2012) Challenges and progress towards understanding the role of effectors in plant-fungal interactions. Curr Opin Plant Biol 15: 477–482. - PubMed
-
- Kader JC (1996) Lipid-Transfer Proteins in Plants. Annu Rev Plant Physiol Plant Mol Biol 47: 627–654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

