Tracking single cells in live animals using a photoconvertible near-infrared cell membrane label
- PMID: 23990881
- PMCID: PMC3753322
- DOI: 10.1371/journal.pone.0069257
Tracking single cells in live animals using a photoconvertible near-infrared cell membrane label
Erratum in
- PLoS One. 2013;8(11). doi:10.1371/annotation/c05446df-ee55-4072-940c-543adff42086. Spencer, Joel A [added]
Abstract
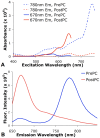
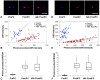
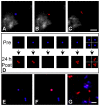
We describe a novel photoconversion technique to track individual cells in vivo using a commercial lipophilic membrane dye, DiR. We show that DiR exhibits a permanent fluorescence emission shift (photoconversion) after light exposure and does not reacquire the original color over time. Ratiometric imaging can be used to distinguish photoconverted from non-converted cells with high sensitivity. Combining the use of this photoconvertible dye with intravital microscopy, we tracked the division of individual hematopoietic stem/progenitor cells within the calvarium bone marrow of live mice. We also studied the peripheral differentiation of individual T cells by tracking the gain or loss of FoxP3-GFP expression, a marker of the immune suppressive function of CD4(+) T cells. With the near-infrared photoconvertible membrane dye, the entire visible spectral range is available for simultaneous use with other fluorescent proteins to monitor gene expression or to trace cell lineage commitment in vivo with high spatial and temporal resolution.
Conflict of interest statement
Figures



References
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, et al. (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846. - PubMed
-
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121. - PubMed
-
- Zhang J, Niu C, Ye L, Huang H, He X, et al. (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL97794/HL/NHLBI NIH HHS/United States
- HL097748/HL/NHLBI NIH HHS/United States
- HL100402/HL/NHLBI NIH HHS/United States
- P50 CA086355/CA/NCI NIH HHS/United States
- MR/J006742/1/MRC_/Medical Research Council/United Kingdom
- P01 AI041521/AI/NIAID NIH HHS/United States
- U01 HL100402/HL/NHLBI NIH HHS/United States
- R01 HL097794/HL/NHLBI NIH HHS/United States
- P01 AI073748/AI/NIAID NIH HHS/United States
- 5 PO1 AI041521-12/AI/NIAID NIH HHS/United States
- CA086355/CA/NCI NIH HHS/United States
- R01 HL097748/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

