The biological clock is regulated by adrenergic signaling in brown fat but is dispensable for cold-induced thermogenesis
- PMID: 23990898
- PMCID: PMC3753278
- DOI: 10.1371/journal.pone.0070109
The biological clock is regulated by adrenergic signaling in brown fat but is dispensable for cold-induced thermogenesis
Abstract
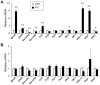
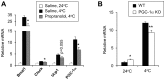
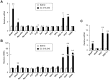
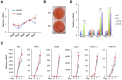
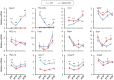
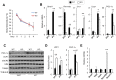
The biological clock plays an important role in integrating nutrient and energy metabolism with other cellular processes. Previous studies have demonstrated that core clock genes are rhythmically expressed in peripheral tissues, including the liver, skeletal muscle, pancreatic islets, and white and brown adipose tissues. These peripheral clocks are entrained by physiological cues, thereby aligning the circadian pacemaker to tissue functions. The mechanisms that regulate brown adipose tissue clock in response to physiological signals remain poorly understood. Here we found that the expression of core clock genes is highly responsive to cold exposure in brown fat, but not in white fat. This cold-inducible regulation of the clock network is mediated by adrenergic receptor activation and the transcriptional coactivator PGC-1α. Brown adipocytes in mice lacking a functional clock contain large lipid droplets accompanied by dysregulation of genes involved in lipid metabolism and adaptive thermogenesis. Paradoxically, the "clockless" mice were competent in maintaining core body temperature during cold exposure. These studies elucidated the presence of adrenergic receptor/clock crosstalk that appears to be required for normal thermogenic gene expression in brown fat.
Conflict of interest statement
Figures

References
-
- Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359. - PubMed
-
- Nedergaard J, Bengtsson T, Cannon B (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–452. - PubMed
-
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, et al. (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

