Suppressor of Cytokine Signaling (SOCS) 5 utilises distinct domains for regulation of JAK1 and interaction with the adaptor protein Shc-1
- PMID: 23990909
- PMCID: PMC3749136
- DOI: 10.1371/journal.pone.0070536
Suppressor of Cytokine Signaling (SOCS) 5 utilises distinct domains for regulation of JAK1 and interaction with the adaptor protein Shc-1
Abstract
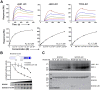
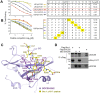
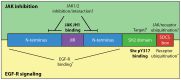
Suppressor of Cytokine Signaling (SOCS)5 is thought to act as a tumour suppressor through negative regulation of JAK/STAT and epidermal growth factor (EGF) signaling. However, the mechanism/s by which SOCS5 acts on these two distinct pathways is unclear. We show for the first time that SOCS5 can interact directly with JAK via a unique, conserved region in its N-terminus, which we have termed the JAK interaction region (JIR). Co-expression of SOCS5 was able to specifically reduce JAK1 and JAK2 (but not JAK3 or TYK2) autophosphorylation and this function required both the conserved JIR and additional sequences within the long SOCS5 N-terminal region. We further demonstrate that SOCS5 can directly inhibit JAK1 kinase activity, although its mechanism of action appears distinct from that of SOCS1 and SOCS3. In addition, we identify phosphoTyr317 in Shc-1 as a high-affinity substrate for the SOCS5-SH2 domain and suggest that SOCS5 may negatively regulate EGF and growth factor-driven Shc-1 signaling by binding to this site. These findings suggest that different domains in SOCS5 contribute to two distinct mechanisms for regulation of cytokine and growth factor signaling.
Conflict of interest statement
Figures


References
-
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. - PubMed
-
- Gan HK, Burgess AW, Clayton AH, Scott AM (2012) Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res 72: 2924–2930. - PubMed
-
- Mullighan CG (2012) The molecular genetic makeup of acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2012: 389–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

