C1 esterase inhibitor reduces lower extremity ischemia/reperfusion injury and associated lung damage
- PMID: 23991040
- PMCID: PMC3753343
- DOI: 10.1371/journal.pone.0072059
C1 esterase inhibitor reduces lower extremity ischemia/reperfusion injury and associated lung damage
Abstract
Background: Ischemia/reperfusion injury of lower extremities and associated lung damage may result from thrombotic occlusion, embolism, trauma, or surgical intervention with prolonged ischemia and subsequent restoration of blood flow. This clinical entity is characterized by high morbidity and mortality. Deprivation of blood supply leads to molecular and structural changes in the affected tissue. Upon reperfusion inflammatory cascades are activated causing tissue injury. We therefore tested preoperative treatment for prevention of reperfusion injury by using C1 esterase inhibitor (C1 INH).
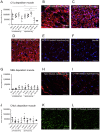
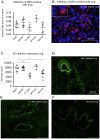
Methods and findings: Wistar rats systemically pretreated with C1 INH (n = 6), APT070 (a membrane-targeted myristoylated peptidyl construct derived from human complement receptor 1, n = 4), vehicle (n = 7), or NaCl (n = 8) were subjected to 3h hind limb ischemia and 24h reperfusion. The femoral artery was clamped and a tourniquet placed under maintenance of a venous return. C1 INH treated rats showed significantly less edema in muscle (P<0.001) and lung and improved muscle viability (P<0.001) compared to controls and APT070. C1 INH prevented up-regulation of bradykinin receptor b1 (P<0.05) and VE-cadherin (P<0.01), reduced apoptosis (P<0.001) and fibrin deposition (P<0.01) and decreased plasma levels of pro-inflammatory cytokines, whereas deposition of complement components was not significantly reduced in the reperfused muscle.
Conclusions: C1 INH reduced edema formation locally in reperfused muscle as well as in lung, and improved muscle viability. C1 INH did not primarily act via inhibition of the complement system, but via the kinin and coagulation cascade. APT070 did not show beneficial effects in this model, despite potent inhibition of complement activation. Taken together, C1 INH might be a promising therapy to reduce peripheral ischemia/reperfusion injury and distant lung damage in complex and prolonged surgical interventions requiring tourniquet application.
Conflict of interest statement
Figures






References
-
- Cooper NR (1985) The classical complement pathway: activation and regulation of the first complement component. Adv Immunol 37: 151–216. - PubMed
-
- McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, et al. (2006) Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology 211: 759–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

