RasGRPs are targets of the anti-cancer agent ingenol-3-angelate
- PMID: 23991094
- PMCID: PMC3749120
- DOI: 10.1371/journal.pone.0072331
RasGRPs are targets of the anti-cancer agent ingenol-3-angelate
Abstract
Ingenol-3-angelate (I3A) is a non-tumor promoting phorbol ester-like compound identified in the sap of Euphoria peplus. Similar to tumor promoting phorbol esters, I3A is a diacylglycerol (DAG) analogue that binds with high affinity to the C1 domains of PKCs, recruits PKCs to cellular membranes and promotes enzyme activation. Numerous anti-cancer activities have been attributed to I3A and ascribed to I3A's effects on PKCs. We show here that I3A also binds to and activates members of the RasGRP family of Ras activators leading to robust elevation of Ras-GTP and engagement of the Raf-Mek-Erk kinase cascade. In response to I3A, recombinant proteins consisting of GFP fused separately to full-length RasGRP1 and RasGRP3 were rapidly recruited to cell membranes, consistent with direct binding of the compound to RasGRP's C1 domain. In the case of RasGRP3, IA3 treatment led to positive regulatory phosphorylation on T133 and activation of the candidate regulatory kinase PKCδ. I3A treatment of select B non-Hodgkin's lymphoma cell lines resulted in quantitative and qualitative changes in Bcl-2 family member proteins and induction of apoptosis, as previously demonstrated with the DAG analogue bryostatin 1 and its synthetic analogue pico. Our results offer further insights into the anticancer properties of I3A, support the idea that RasGRPs represent potential cancer therapeutic targets along with PKC, and expand the known range of ligands for RasGRP regulation.
Conflict of interest statement
Figures

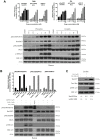

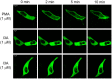
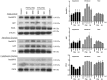
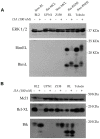
References
-
- Ogbourne SM, Hampson P, Lord JM, Parsons P, De Witte PA, et al. (2007) Proceedings of the First International Conference on PEP005. Anticancer Drugs 18: 357–362. - PubMed
-
- Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, et al. (2012) Ingenol mebutate gel for actinic keratosis. N. Engl. J. Med. 366: 1010–1019. - PubMed
-
- Ogbourne SM, Suhrbier A, Jones B, Cozzi SJ, Boyle GM, et al. (2004) Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res 64: 2833–2839. - PubMed
-
- Hampson P, Chahal H, Khanim F, Hayden R, Mulder A, et al. (2005) PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood 106: 1362–1368. - PubMed
-
- Mason SA, Cozzi SJ, Pierce CJ, Pavey SJ, Parsons PG, et al. (2010) The induction of senescence-like growth arrest by protein kinase C-activating diterpene esters in solid tumor cells. Invest New Drugs 28: 575–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

