Protein tyrosine phosphatase non-receptor type 22 modulates NOD2-induced cytokine release and autophagy
- PMID: 23991106
- PMCID: PMC3753240
- DOI: 10.1371/journal.pone.0072384
Protein tyrosine phosphatase non-receptor type 22 modulates NOD2-induced cytokine release and autophagy
Abstract
Background: Variations within the gene locus encoding protein tyrosine phosphatase non-receptor type 22 (PTPN22) are associated with the risk to develop inflammatory bowel disease (IBD). PTPN22 is involved in the regulation of T- and B-cell receptor signaling, but although it is highly expressed in innate immune cells, its function in other signaling pathways is less clear. Here, we study whether loss of PTPN22 controls muramyl-dipeptide (MDP)-induced signaling and effects in immune cells.
Material & methods: Stable knockdown of PTPN22 was induced in THP-1 cells by shRNA transduction prior to stimulation with the NOD2 ligand MDP. Cells were analyzed for signaling protein activation and mRNA expression by Western blot and quantitative PCR; cytokine secretion was assessed by ELISA, autophagosome induction by Western blot and immunofluorescence staining. Bone marrow derived dendritic cells (BMDC) were obtained from PTPN22 knockout mice or wild-type animals.
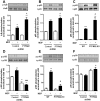
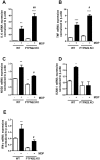
Results: MDP-treatment induced PTPN22 expression and activity in human and mouse cells. Knockdown of PTPN22 enhanced MDP-induced activation of mitogen-activated protein kinase (MAPK)-isoforms p38 and c-Jun N-terminal kinase as well as canonical NF-κB signaling molecules in THP-1 cells and BMDC derived from PTPN22 knockout mice. Loss of PTPN22 enhanced mRNA levels and secretion of interleukin (IL)-6, IL-8 and TNF in THP-1 cells and PTPN22 knockout BMDC. Additionally, loss of PTPN22 resulted in increased, MDP-mediated autophagy in human and mouse cells.
Conclusions: Our data demonstrate that PTPN22 controls NOD2 signaling, and loss of PTPN22 renders monocytes more reactive towards bacterial products, what might explain the association of PTPN22 variants with IBD pathogenesis.
Conflict of interest statement
Figures






References
-
- Chen G, Shaw MH, Kim YG, Nunez G (2009) NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol 4: 365–398. - PubMed
-
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, et al. (2003) Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278: 8869–8872. - PubMed
-
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, et al. (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411: 603–606. - PubMed
-
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, et al. (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411: 599–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

